The media is falsely claiming the results of a newly published study — which included only a dozen women and whose lead author has been funded by the abortion pill manufacturer, DANCO Laboratories — somehow concludes that abortion pill reversal treatment is dangerous for women.
The study, “Mifepristone Antagonization With Progesterone to Prevent Medical Abortion,” was published by the Journal of Obstetrics and Gynecology, commonly referred to as “The Green Journal,” which is the official publication of the pro-abortion American College of Obstetricians and Gynecologists (ACOG). As Live Action News previously pointed out, the study is the result of a failed clinical trial published under the name “Blocking Mifepristone Action With Progesterone.” It was sponsored by the University of California-Davis and the Society of Family Planning, both of which have strong ties to abortion. The study was terminated sometime between August and September 2019.

Creinin study on abortion pill reversal published in AJOG
The study intended to recruit 40 pregnant participants to take the first of the two pills (Mifepristone) in the approved abortion pill regimen at either the University of California-Davis or at Planned Parenthood Mar Monte. Then, pregnant participants were given either progesterone treatments to try to save their preborn children… or a placebo. The mere suggestion of using pregnant women in this kind of study raises ethical concerns — however, the authors claim that the “University of California, Davis, Institutional Review Board approved this study.” The study was abruptly suspended and later terminated after enrolling just 12 women, following three instances of “severe hemorrhage requiring ambulance transport to hospital.”
But instead of being critical of the unethical experiment by abortion enthusiasts, the media is giving the authors cover by claiming the study is proof that treating women with progesterone to “reverse” a chemical abortion might be dangerous. This is beyond dishonest.

Vice misrepresents Creinin study on abortion pill reversal
The study’s extremely small sample size, which dwindled from 40 to just 12 women (five of whom were African American) reports the following:
12 enrolled – two patients dropped out:
- One patient (placebo) requested suction aspiration due to “increased anxiety about bleeding that started 2 days after mifepristone [abortion pill] use.”
- One patient (progesterone) “developed increasing nausea and vomiting after enrolling” and eventually requested a suction aspiration.
Progesterone: 5 patients
- Four patients taking progesterone had continuing pregnancies at two weeks.
- One patient “started having brisk bleeding and called an ambulance.” The ER “found no gestational sac and a heterogenous endometrial lining of approximately 1.5 cm. Heavy bleeding lasted about 3 hours overall, and no intervention was needed.”
Placebo: 5 patients
- Two patients had continuing pregnancies at two weeks.
- One patient had no gestational cardiac activity three days after mifepristone use and had an uneventful suction aspiration.
- One patient “called an ambulance after onset of heavy vaginal bleeding” following taking of mifepristone (abortion pill). She eventually had a suction abortion.
- One patient “called an ambulance after experiencing hemorrhage” after taking mifepristone and she “received a 1-unit transfusion of packed red blood cells.”
Yet the media fails to point out:
- Twice as many patients who had progesterone had a continued pregnancy — ironically confirmed by “gestational cardiac activity,” despite claims by abortion advocates the baby in womb doesn’t have a heartbeat at this stage.
- Twice as many patients transported by ambulance for bleeding took the placebo over progesterone, while another who took the placebo appears to have lost the baby within days of taking mifepristone.
- Bleeding, nausea, cramping, are known “risks” for abortion pill.
Despite clearly stating in the study, “This level of evidence is inadequate to support or refute the benefits and risks of any treatment,” media outlets are pouncing, with some falsely claiming APR treatments are dangerous for women. And yet, after news several women died during clinical trials of the abortion pill, the media failed to label abortion pill as dangerous.
Connections to abortion pill manufacturer DANCO and its investors:
The study’s principal investigator and lead author was Mitchell D. Creinin, MD, who previously participated in initial clinical trials of the abortion pill, RU-486.
NPR spoke to Creinin and noted that he “acknowledges that his study was limited by its premature termination and small sample size,” then added that Creinin “hypothesizes that taking mifepristone without misoprostol may be especially risky later in the first trimester of pregnancy. All three patients with severe bleeding were at least 56 days into their pregnancies.”
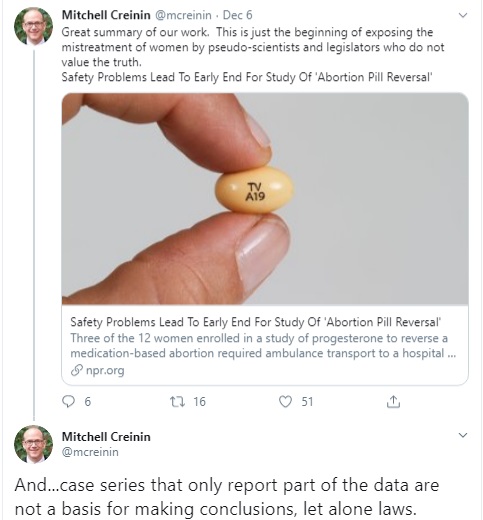
Mitchell D Creinin tweets against APR (Image: Twitter)
Creinin has a number of conflicts the media selectively left out. While Creinin acknowledged in the study that he is a “consultant for DANCO Laboratories,” Live Action News has also documented that…
- Creinin has long history with abortion
- Creinin serves as consultant for abortion pill (Mifeprex) manufacturer DANCO and receives consulting fees and compensation from the company.
- Creinin receives an honorarium from DANCO.
- Creinin provides “third-party telephone consults” for DANCO.
- Creinin’s study sponsor is financed by the Packard Foundation, one of DANCO’s major financial investors.
- Creinin is a founding member of the Society of Family Planning, which was collaborating on the study. This organization’s mission is to support abortion research, and it is funded by the Packard Foundation, a large investor in DANCO.

Mitchell D Creinin financial conflicts from Danco abortion pill MFG APR Study

Mitchell Creinin consultant for Danco abortion pill MFG

Mitchell Creinin third party consults for Danco abortion pill MFG
Author Laura Dalton is listed as “Chief Medical Officer” of Planned Parenthood Mar Monte’s managing board.
Author Rachel Steward, MD, is connected to FPA Women’s Health, which commits abortions. Both FPA and “Rachel Steward” are mentioned in the Center for Medical Progress undercover investigations (transcript here).
University of California Davis:
- Packard (DANCO’s investor) funds UC Davis and has for years.
- UCD provides abortions at Division of Family Planning.
- UCD was location site for study as well as clinical trial on pharmacy dispension of abortion pill, conducted by abortionist Daniel Grossman.
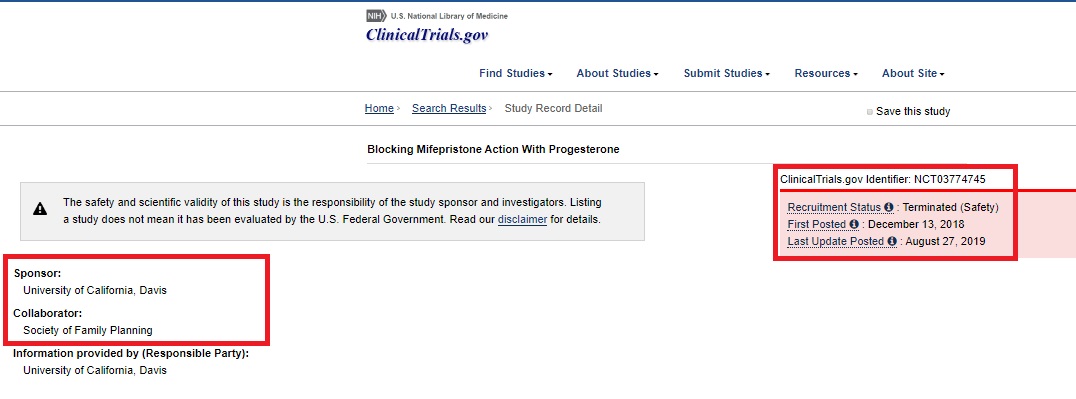
Mitchell D Creinin clinical trial on abortion pill terminated
Society of Family Planning (SFP):
- Study supported by Society of Family Planning Research Fund, arm of Society of Family Planning (SFP), which granted UC Davis $401,764.
- Live Action News previously documented that SFP was founded in 2005 with generous contribution from Packard Foundation, original investor of DANCO Laboratories, a regular exhibitor at SFP events.
- SFP funded by DANCO investor, the Buffett Foundation (ex: 2011, 2014, 2016) — as in billionaire Warren Buffett.
- Buffett funds the Population Council, a eugenics-founded organization responsible for bringing the abortion pill into the U.S.
- Buffett funds the University of California, granting them $78 million.
The abortion pill reversal website is operated by Heartbeat International. Its Director of Medical Impact, Christa Brown, told Live Action News, “This study actually showed that the abortion pill, not reversal, carries major health risks to a mother.”
“Well over 900 women have successfully stopped an abortion and saved their children through abortion pill reversal. This life-saving intervention is a cutting-edge application of a time-tested, FDA-approved treatment used for decades to prevent miscarriage and preterm birth. It involves emergency, ongoing doses of progesterone to counteract the effects of the first abortion pill,” she said.
In a published statement, Dr. Tara Sander Lee, Charlotte Lozier’s Senior Fellow and Director of Life Sciences, also weighed in, writing, “Long before this study was published, it was a known fact that Mifeprex can cause serious and sometimes fatal infections, excessive bleeding, and incomplete termination requiring follow-up surgery. This study does nothing but further prove these serious, life-threatening risks when taking the abortion pill.”
“Like” Live Action News on Facebook for more pro-life news and commentary!







