Congressional leaders have issued a letter of concern over what they refer to as “an unethical chemical abortion trial” which is using the abortion pill to end second trimester pregnancies in minority women in the West African nation of Burkina Faso.
The letter, dated February 7, 2020, was addressed to Health and Human Services (HHS) Secretary Alex Azar and FDA Commissioner Stephen Hahn, and signed by Reps. Mark Meadows (NC-11), Ron Estes (KS-4), and Chris Smith (NJ-4), as well as 29 additional colleagues. It reads, in part:
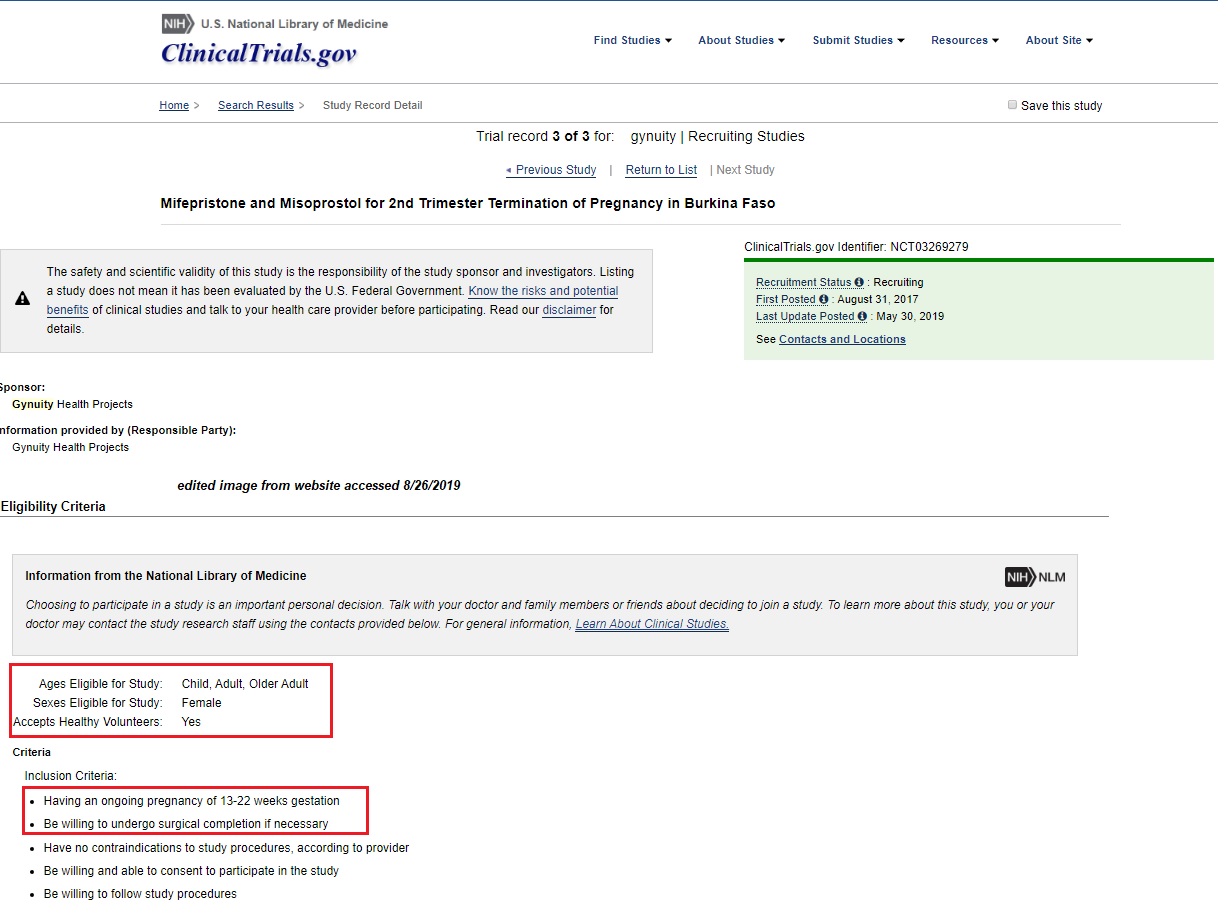
The study recruited Burkinabe women who were 13-22 weeks pregnant—the second trimester—to test the effectiveness of mifepristone and misoprostol for an abortion…We find it troubling that Burkina Faso was selected as the location for this clinical trial, where poor health and safety conditions endanger the participants involved. Burkina faces a severe lack of medical professionals….

Congressional letter regarding abortion pill clinical trial Feb 7 2020
Charlotte Lozier Associate Scholar Dr. Christina Francis reacted to the trial, saying, “In general, throughout Africa, regardless of whether you’re in this big city or a small village, blood products tend to be difficult to come by. There are national shortages very frequently…. And so, even if you’ve got a woman in, say, the biggest hospital in the capital of Burkina Faso, that doesn’t mean that she’s going to have access to blood products just because she’s in that hospital.”
Given that one of the possible adverse effects of the pill is heavy bleeding, the Congressional letter goes on to state, “[…]Africa also faces frequent blood shortages, and the clinical trial’s director, Dr. Blandine Thieba, even acknowledged the dire need for blood bags in the country during the study.”
In other words, if something goes wrong during the trial, Burkina Faso isn’t equipped to deal with the consequences.
The clinical trial began recruiting in August of 2017 and is being sponsored by Gynuity Health Projects. The abortion pill — Mifeprex combined with a second drug called Misoprostol — has only been approved in the U.S. by the FDA to end an early pregnancy up to 10 weeks or one which is 70 days or less since the woman’s first day of the last menstrual period. Live Action News previously documented how, in this clinical trial, Gynuity is recruiting children and adult women for late-term abortions from 13-22 weeks.
Abortion Pill Clinical Trial outside US for second trimester sponsored by Gynuity (Image: FDA Clinical Trial website)
Gynuity, founded by Beverly Winikoff, M.D., M.P.H, who had a previous contract with abortion pill manufacturer DANCO Laboratories, has been aggressively working to expand abortion and has also been sponsoring U.S. abortion pill clinical trials via TelAbortion.
Winikoff was previously employed at the Population Council, which brought the abortion pill into the U.S. and set up DANCO. As previously documented, the Population Council has been heavily steeped in eugenics. In addition, the abortion pill, formerly called RU-486, has ties to the manufacturer of the deadly gas Zyklon-B, used by the Nazis during the Holocaust.
Gynuity has other concerning connections:
- Gynuity is funded (see here) by DANCO’s original business investor, the Packard Foundation, as well as the Buffett Foundation and the Population Council.
- Gynuity is funded by Ibis Reproductive Health (see 2017 990 report), an organization directly funded by DANCO. Gynuity’s “menstrual regulation” experiments as well as their TelAbortion clinical trial will take place at Packard-funded Carafem abortion facilities.
- Gynuity has partnered with Packard and Buffett funded Planned Parenthood in their their TelAbortion clinical trial.
- Packard’s money trail to Gynuity on their own website appears to have been mysteriously scrubbed, but remains online in a cached version.
ACI Africa Editor-in-Chief Fr. Don Bosco Onyalla, part of a coalition of pro-life activists exposing the move, called the trial “immoral and unethical” and told EWTN the group is “taking advantage of African [women]…It is a way of promoting the culture of death in Africa and trying to limit populations when abortion is illegal in that very country….”
Onyalla even questioned whether bribery may have been involved in getting the trial into that country, saying, “The law does not permit abortion. It is interesting to find out how this company was able to find a way of entry into the country….”
A Tweet thread published by the Charlotte Lozier Institute addressed the trial as well, writing:
“[…T]he most common adverse effect is hemorrhaging. And in a country where blood is in short supply, this can be a dangerous, potential deadly consequence…This study reflects the dark history of the abortion industry’s involvement in population control efforts abroad in developing countries such as Bangladesh and India.”
The Congressional letter urged the FDA “to carefully review this trial,” noting, “We are deeply concerned that this trial was not conducted in accordance with good clinical practices…. We are seeking clarification on whether this trial was approved by the Institutional Review Board (IRB)….”
Lawmakers noted an additional concern that the move may have violated this FDA Statute, by unnecessarily exposing the pregnant women to risk, let alone their preborn children.

Congressional letter regarding abortion pill clinical trial Feb 7 2020 part b
“I am deeply concerned that a group would so blatantly put vulnerable women in a developing country in danger under the guise of ‘maternal health’ for the pursuit of expanded profits for the abortion industry. Tactics like this used by Gynuity, and others within the industry, are reprehensible, and my colleagues and I remain committed to holding groups like this accountable for their unethical actions abroad,”Rep. Meadows wrote in a press release.
“Like” Live Action News on Facebook for more pro-life news and commentary!







