The abortion industry does not look favorably upon regulations. Examples of this can be found in its years of litigation against every kind of regulation, including parental notification laws for minors seeking abortions, 24-hour waiting periods, and informed consent measures. Now, the manufacturer of the abortion pill — which is tasked with upholding Food and Drug Administration’s (FDA) safety requirements, certifying prescribers, and removing violators — is inexplicably funding an abortion training curriculum which openly contradicts the FDA’s safety standards for the drug.
Abortion pill manufacturer Danco Laboratories has given an “unrestricted educational grant” to the TEACH Early Abortion Training Curriculum, which openly promotes self-managed (DIY) abortion, illegal abortion pill websites, and later gestational limits, in direct vioilation of FDA safety measures called REMS. Under REMS, Danco is required to “ensure that healthcare providers who prescribe their mifepristone [abortion pill] are specially certified in accordance with the requirements.” The manufacturer must also “de-certify healthcare providers who do not maintain compliance with certification requirements.”
The TEACH Early Abortion Training Curriculum was established in 2003 with financial help from the Packard Foundation, one of Danco’s original funders. The curriculum’s website notes:
In 2002, the Bixby Center launched the Advancing New Standards in Reproductive Health (ANSIRH) Program…to enhance access to abortion. With seed funding from the Packard Foundation, ANSIRH established a Planned Parenthood training site for motivated residents and practicing physicians to learn early abortion procedures. In July 2003, support from an anonymous donor allowed the Center to expand its training initiatives by pioneering the TEACH Project…
TEACH acknowledges receiving “[s]upport, sponsorship, staffing and/or collaboration” from the National Abortion Federation, Gynuity Health Projects, ANSIRH, Planned Parenthood, the Society of Family Planning (SFP), Danco, and others. The curriculum’s website admits that it “receives unrestricted educational grant funding from Danco, LLC,” and has for years.
TEACH flouts multiple FDA requirements, as seen below.
LATER GESTATIONAL LIMITS
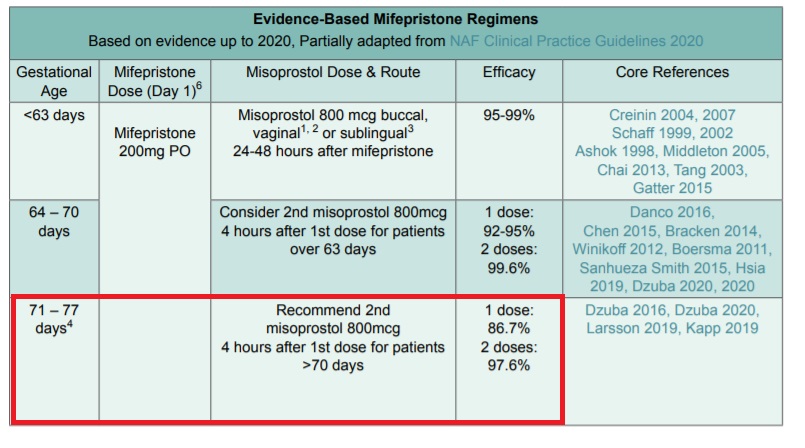
The abortion pill regimen — which consists of the drugs mifepristone (also called Mifeprex) and misoprostol — has only been approved by the FDA for abortion up to 10 weeks of pregnancy (70 days). Yet this Danco-funded abortion training curriculum recommends medication abortion through 11 weeks (77 days), beyond the FDA-approved limit.
It is important to note that as pregnancy progresses, the failure rate of the abortion pill increases, as does the risk of complications.
TEACH abortion training Medication abortion at 11 weeks evidence based regimen
The TEACH curriculum suggests abortion counselors should be selective in how they sell abortion to clients. TEACH recommends against saying, “Your baby is eight weeks old,” and instead refers to the preborn child as “a pregnancy.” This recommendation matches the testimonies of many former Planned Parenthood workers.
A medication abortion will most likely result in the child being expelled by the woman at home, possibly alone. TEACH suggests that, “If the patient is anxious about seeing the pregnancy or fetal tissue, consider showing a drawing… If they are not comfortable, they may prefer an aspiration abortion.”
A drawing would likely not prepare a woman for the potential trauma of seeing the recognizably human shape of her child as he or she is expelled from the womb.
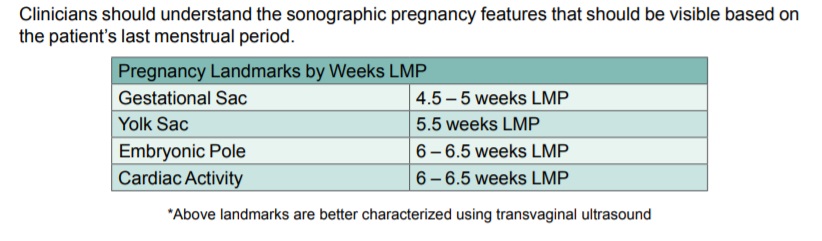
The TEACH manual admits that an embryo at 6 – 6.5 weeks LMP has cardiac activity and “grows 1 mm per day thereafter until 12-14 weeks.” (A preborn child’s heart begins to beat between 16 and 21 days after fertilization.) The abortion curriculum also identifies how an “[a]bsence of embryo with heartbeat,” is potentially a sign of early pregnancy loss.
TEACH abortion training gestational landmarks
SELF-MANAGED ABORTION
TEACH describes a self-managed abortion (SMA) as “the use of pills for abortion without the oversight by a licensed clinician.” But under the FDA’s safety protocol, “Mifepristone must be dispensed to patients only in certain healthcare settings, specifically clinics, medical offices, and hospitals, by or under the supervision of a certified prescriber.”
TEACH even acknowledged the FDA’s requirement, but stated that “Mifepristone can safely be taken at home though U.S. regulation still requires it be dispensed in a clinical setting by a clinician.”
UNAPPROVED ABORTION METHODS
The FDA has approved the previously mentioned abortion pill regimen of mifepristone and misoprostol. Yet, according to TEACH, “self-managed abortion” can also include methods unapproved by the FDA, such as “misoprostol alone” as well as “herbs and objects or substances inserted into the vagina or cervix” and “deep abdomen massage.”
“Misoprostol-only is a reasonable alternative to mifepristone-containing regimens when mifepristone is not available, though it is less effective,” the TEACH curriculum states.
ILLEGAL ABORTION PILL WEBSITES
Under self-managed abortion, the manual lists as “resources” two websites working overseas to ship abortion pills globally and into the United States illegally: Aid Access and Women on Web. The FDA previously sent clear warnings to websites that ship abortion pills illegally into the U.S.
And yet, this abortion training manual funded by the abortion pill’s manufacturer offers no such warning.

TEACH abortion training Medication abortion promotes websites illegally distributing the abortion pill
LABS UNNECESSARY
Under the FDA’s REMS safety protocol, prescribers of the abortion pill must be able to “assess the duration of pregnancy accurately” and to “diagnose ectopic pregnancies.” Yet, TEACH suggests lab work is unnecessary unless the clinician suspects a need for it.
“No labs are required unless: Rh if pregnancy dating >56 days and unknown Rh,” TEACH writes.
TEACH also appears to have adopted the no-test abortion protocol, writing, “In early first trimester (<70 days) pregnancy, LMP alone has been shown to be an accurate means of estimating gestational age with low rates of under- or overestimation in abortion evaluation to mid first trimester or 63 days.”
“Pairing bimanual exam with LMP dating may increase the accuracy of gestational age estimation,” TEACH writes, but emphasizes that such an exam “is not required to proceed with a medication….” The manual notes that “if there are any signs or symptoms of ectopic pregnancy, an ultrasound may be warranted.”
“Accurate pregnancy dating is a key component of the pre-abortion evaluation… When pregnancy dating cannot be determined by last menstrual period, ultrasound can be used. Ultrasound aids in pregnancy dating and the detection of abnormal pregnancy including ectopic pregnancy and early pregnancy loss,” TEACH writes. However, the abortion training manual goes on to claim that “[r]emoving unnecessary labs and visits can improve access and patient experience, without jeopardizing safety.”
SURGICAL INTERVENTION SKILLS UNNECESSARY
According to the FDA’s REMS safety protocol, abortion pill prescribers must be able to “provide surgical intervention in cases of incomplete abortion or severe bleeding, or to have made plans to provide such care through others, and ability to assure patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.”
Yet, according to the Danco-funded TEACH curriculum, “Medication abortion [abortion pill] is relatively easy to integrate into clinical practice, expands access to abortion care, and can be provided in settings without ultrasound or ability to provide uterine aspiration with appropriate referral option if needed.”
TEACH is published by UCSF’s Bixby Center for Global Reproductive Health and boasts contributors and authors associated with UCSF as well as Planned Parenthood. Advisory committee members include Dr. Daniel Grossman and Alice Mark, a medical director for the National Abortion Federation.
“Like” Live Action News on Facebook for more pro-life news and commentary!







