A petition from 48 pro-abortion groups is asking the U.S. Food and Drug Administration (FDA) to approve the abortion pill (mifepristone) for miscarriage care — a move that is essentially a scheme to get the drug approved under “other indications” and circumvent laws that restrict abortion. In addition, the petition is using the ruse to convince the FDA to remove mifepristone from an important safety system known as the Risk Evaluation and Mitigation System (REMS).
The letter was signed by the pro-abortion American College of Obstetricians and Gynecologists (ACOG), the American Humanist Association, American Medical Association (AMA), Expanding Medication Abortion Access (EMAA) Project, NARAL Pro-Choice America, the National Abortion Federation (NAF), Physicians for Reproductive Health (PRH), Planned Parenthood Federation of America (PPFA), and UCSF Bixby Center for Global Reproductive Health, among others who could likely benefit financially from the move. It read in part:
The American College of Obstetricians and Gynecologists submits this petition on behalf of itself and 48 other organizations listed…request that the Food & Drug Administration (FDA) ask Danco Laboratories, LLC… the holder of the approved new drug application for Mifeprex (mifepristone)—to submit a Supplemental New Drug Application (sNDA) that seeks to add miscarriage management as an indication to the drug’s label and to eliminate or modify mifepristone’s Risk Evaluation and Mitigation Strategy (REMS) so that it is not unduly burdensome for that use.
In the meantime, Petitioners also request that FDA immediately exercise enforcement discretion with respect to the use and distribution of mifepristone for miscarriage management without complying with the REMS.
The letter also claims that “clinicians who treat miscarriage and their patients have an urgent need to address increasing barriers to accessing mifepristone.”
Since when? Doctors have been treating miscarriage since well before mifepristone was introduced.
The REMS
The abortion advocates then “urge both FDA and Danco to act expeditiously” and “request that FDA immediately announce that it will exercise enforcement discretion to permit the use and distribution of mifepristone consistent with the requested miscarriage indication and changes in the REMS for this indication.”
The REMS limits prescribers to only those approved by Danco or the generic manufacturer GenBioPro — but removing the REMS for miscarriage care would open access to the abortion pill to anyone and expand abortion in the process.
The FDA approved the drug mifepristone (which starves the preborn child of nutrients) as the abortion pill in 2000, in a regimen along with a second drug, misoprostol (which causes contractions to expel the baby). It was later placed under an FDA safety system known as REMS, after multiple deaths prompted a closer watch of the drug.
Recently, the FDA under the Biden administration has chosen to weaken the REMS and allow the deadly drugs to be shipped by mail, all while the abortion industry recklessly implemented a “no-test” protocol. This enabled abortion facilities to maximize profits by dispensing abortion pills without conducting testing, labs, or ultrasounds to determine gestational age or rule out life-threatening ectopic pregnancies. This potentially places women’s lives at greater risk.
The drug regimen has only been approved through 10 weeks (70 days) gestation, yet the unregulated abortion industry is openly defying that gestational limit and selling the abortion pill well into the second trimester of pregnancy — when the failure rate drastically increases. They know that when a failure occurs, women who “self-manage” abortions will (upon the advice of abortion insiders) either return to the abortion facility or present to the emergency room where they have been instructed to claim they are experiencing a natural miscarriage. There, ER doctors are being encouraged to falsify medical records by the likes of Dr. Daniel Grossman, a prominent abortionist cited in the group’s FDA petition.
The Ruse
While the petition has been proposed under the guise of medicine, the real reason — it would appear — is to make the abortion drug easier to dispense in states where abortion is restricted or illegal, and where the abortion pill could ultimately be banned. And the ruse is not a new one to the industry.
Case in point: in 2020, Gynuity Health Projects (GHP), which has been conducting multiple clinical trials to expand the abortion pill and also signed the letter, took part in a webinar where they explicitly detailed a back-door plan to get the abortion drug into countries where abortion is illegal by having the abortion pill approved for other indications.
Gynuity was founded by Beverly Winikoff (who shockingly credited the 9/11 terrorist attacks with saving the abortion pill by drowning out the news of a woman’s abortion pill death) and is heavily funded by investors of the abortion pill manufacturer, Danco Laboratories. Winikoff has served on the board of the NAF (which was at one time directly funded by Danco), as well as the Buffett and Packard Foundations (early investors in Danco).
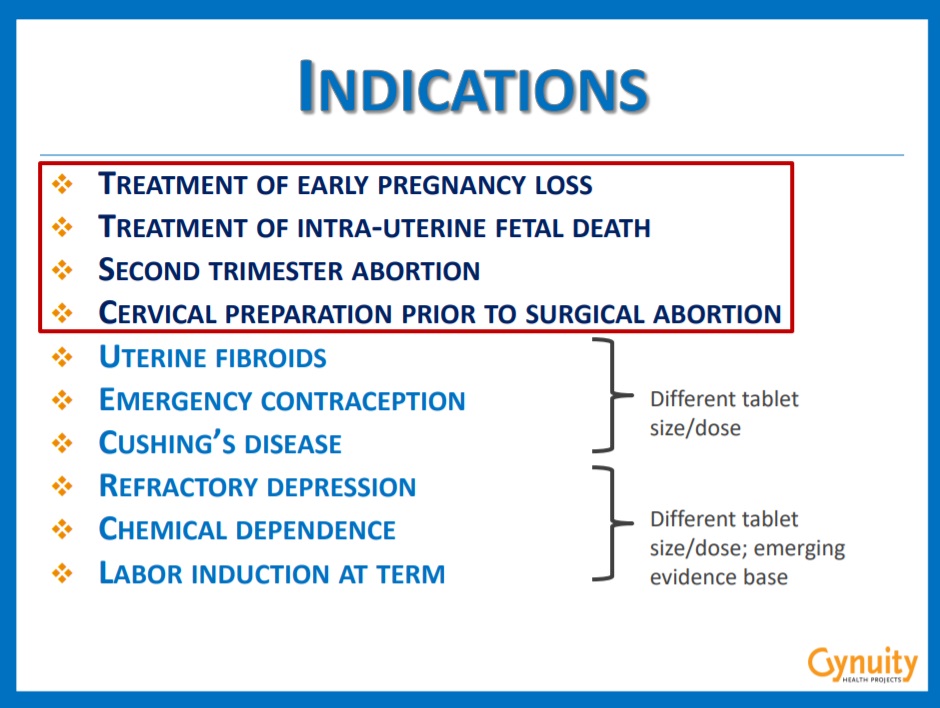
Gynuity webinar Mifepristone indications slide
In the 2020 webinar, Jennifer Blum, Gynuity’s VP for Programs and Strategic Initiatives and former Program Officer for the Packard Foundation — a major early investor in Danco — said in the webinar that registering the abortion pill to treat conditions unrelated to abortion is to “bypass hurdles.”
Gynuity’s Senior Program Associate Laura Frye also joined the webinar, where she introduced a “tangible briefing document” summarizing a list of additional indications that would get mifepristone into pharmacies around the world. The document states (emphasis added):
One strategy to increase availability and market sustainability of mifepristone is to register it for additional indications. Several of these “uncontroversial” indications are legal in most jurisdictions and could provide an entry point for the medication to be listed on national drug registries, stocked in hospitals and other health care facilities, and integrated into health care systems.
In countries where there are highly restricted legal indications for abortion and/or where opposition to abortion on request impacts drug registration regulatory processes, focusing on other indications may eliminate some hurdles to product registration. Additionally, in the face of constrained health care budgets, medications that address multiple indications may be more appealing for purchase by health systems.
The slide show used in the webinar instructs abortion industry pill pushers that “no consensus on which indications might be the most useful. Multiple indications are more likely to result in availability and use of mifepristone.” It went on to state that the indication should be “non-abortion related” so as to “not cause controversy.”

Increasing access to abortion under other indications non abortion related (Gynuity HP webinar slide)
Live Action News previously reported that the webinar was put on by Reproductive Health Supplies Coalition (RHSC) which became involved in the Gates-funded Family Planning 2020 initiative. The coalition includes the Bill and Melinda Gates Foundation, the Bixby Center for Global Reproductive Health, the David and Lucile Packard Foundation, DKT International, GenBioPro, the Guttmacher Institute, Ibis Reproductive Health, Planned Parenthood, and the Population Council.
RHRC’s document for “registering mifepristone for other indications” states in part, “Mifepristone registration for indications other than first trimester abortion has the potential to be a major game changer by creating the framework to facilitate greater commodity access in restricted settings.”
The desire to treat a tragic miscarriage by giving women the exact same pill that kills living preborn babies is a warped gesture which only benefits the abortion industry and those who advocate for its expansion. The abortion pill has been a boon for the industry, which, as of 2020, now makes up more than half (54%) of all abortions committed in the United States.








