FDA Commissioner Dr. Robert Califf appeared before a Senate Agriculture Appropriations Subcommittee hearing on May 8, 2024, where he was grilled about the dangers of the abortion pill. He was specifically asked why the FDA is allowing the drug to be prescribed without “ever seeing a doctor” and why there is a lack of oversight by the Food and Drug Administration (FDA) regarding “advanced provision” and ”stockpiling” of the drug.
The hearing was centered around a review of the FY2025 budget request, where the FDA Commissioner presented a budget of “$7.22 billion, which represents an overall increase of approximately $341 million in annual funding above the FY 2024 Enacted level,” Califf said in his testimony.
But during the hearing, U.S. Senator Cindy Hyde-Smith (R-Miss.), chair of the Senate Pro-Life Caucus, asked Califf questions about the abortion pill, mifepristone.
Califf served as the Obama Administration’s FDA Commissioner from 2016-2017 following his 2015 nomination. Under Califf’s leadership, in 2016, the FDA loosened several modifications to a safety requirement for the abortion pill (Mifeprex/mifepristone) known as the Risk Evaluation and Mitigation System (REMS), which the FDA requires “for certain medications with serious safety concerns.” Only a few medications require REMS.
U.S. Senator Cindy Hyde-Smith grills FDA Commissioner Califf on abortion pill May 2024
“You can get this without doctor oversight”
Senator Hyde-Smith pointed out to Califf that her own husband was required to see his physician in person “for a sinus medication” yet she was perplexed why the abortion pill was available under FDA safety regulations without an in-person visit with a physician.
“Commissioner Califf, I have repeatedly brought your attention to the dangers that women face when allowed dangerous life-ending chemical abortion drugs, which can be ordered by consumers through the mail or purchased in retail pharmacies without ever seeing a doctor in person,” Senator Hyde-Smith said. “So, it blows me away that you can get this with no doctor oversight.”
“The FDA is supposed to protect Americans from taking medications that could be harmful,” she added. “However, you continue to look the other way and claim mifepristone is ‘safe,’ in part, based on the lack of reports of non-fatal adverse events, due to the FDA’s own relaxed reporting requirements, and continue to allow women to put their lives at risk in the name of the pro-abortion agenda” (emphasis added).
Under Califf’s leadership, in 2016, the FDA removed the in-person requirement for prescribing the drug, allowed non-physicians to prescribe the pill, and removed the requirement that all adverse events (complications) be reported to the FDA by the pill’s manufacturer, requiring only deaths to be reported.
A lawsuit recently argued before the U.S. Supreme Court has addressed several of these dangers and is challenging the legitimacy of the FDA’s recent expansions of the abortion pill — likely the most important case since the Dobbs v. Jackson case which overturned Roe v. Wade.
Because Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA) was in the litigation phase, Califf claimed he had to be cautious about his response. “I know you are aware that I’m limited in what I can say because the issues around this are currently under consideration by the Supreme Court,” Califf replied.

FDA Commissioner Robert Califf grilled about advanced provision of abortion pill May 2024
Who can prescribe, and who holds prescribers accountable?
The FDA Commissioner claimed in his response to the Senator that “Companies can’t write prescriptions. It’s doctors. Remember, we don’t regulate the practice of medicine. It has to be a doctor that writes the prescription.”
But Califf is not entirely correct.
Under the FDA’s REMS, the abortion pill can only be prescribed by approved clinicians. This would entail any medical professional (physician, nurse, or midwife etc.) legally permitted by the state in which they are licensed to prescribe a drug. To become a prescriber, all the “clinician” must do is sign the prescriber agreement.
This can be problematic, as a lawsuit filed against Planned Parenthood of Southern New England revealed. It alleged that the assessment of Planned Parenthood’s certified nurse midwife Gannon Ward was so egregiously incorrect that she misdated the late pregnancy of Plaintiff Christin Lafo at just six weeks and three days before selling her an abortion pill. Lafo gave birth two days later in a toilet to a 22-week stillborn baby boy whom she named Kyle Shawn Brady.
The current prescriber agreement (March 2023) states that prescribers must have the “[a]bility to provide surgical intervention in cases of incomplete abortion or severe bleeding or have made plans to provide such care through others and be able to assure patient access to medical facilities equipped to provide blood transfusions and resuscitation, if necessary.”
Those tasked with holding prescribers to these requirements, however, are the ones with the most to gain: the drug’s manufacturers, Danco Laboratories and the generic GenBioPro.
So, while some “clinicians” can legally prescribe medications, they may not be permitted to provide surgeries which might be needed to treat a possible complication. As a result, prescribers may be sending women to emergency rooms if the women feel they are experiencing a complication.

March 2023 FDA prescriber agreement for Mifepristone
The rapid expansion of the abortion pill
In April of 2021, under the guise of the COVID-19 pandemic, the Biden administration’s FDA began to allow limited mail-order pharmacy distribution by expanding the REMS safety regulations. By December of 2021, the Biden FDA had further weakened the REMS by eliminating the in-person dispensing requirement and enabling the abortion pill to be permanently shipped by mail.
Then, in January 2023, the Biden FDA further gutted the REMS by announcing it would allow retail pharmacies to dispense the drug after they have been prescribed. Pharmacy chains CVS and Walgreens among other retail outlets have already begun to dispense the drug.
Despite clear limitations on who can prescribe the drug, Big Abortion is already allowing pharmacists in some states to both prescribe and dispense the abortion pill.
The problem of stockpiling and “advance provision”
Live Action News has documented multiple times how Big Abortion is more than willing to market abortion drugs as “missed period” pills or to ship “advance provision” of the abortion pill in violation of the Food and Drug Administration’s (FDA) REMS safety standards. These standards require prescribers of the drug to be able to properly date an actual pregnancy — a concern pointed out in the hearing.
In addition, Live Action News has previously documented abortion pill stockpiles in multiple states. While mifepristone is the only drug approved by the FDA for the termination of pregnancy, some states are also stockpiling misoprostol, the companion drug used in the abortion pill regimen.
Riddle me this @US_FDA requires prescribers + dispensers MUST be certified by abortion pill mfg Danco/GBP + have ability to provide specific care.
How are states #1) prescribers (clinicians) or dispensers (pharmacies) + able to stockpile abortion pills? https://t.co/shq9nEuS7E
— Carole Novielli (@CaroleNovielli) June 8, 2023
“It has been reported on more than one instance that American women are stockpiling the abortion pills through advanced prescribing through abortion companies and providers,” Senator Hyde-Smith said. “It’s not even approved for that, and they can get it without even seeing a doctor.”
The senator then pointed to a 2022 Politico article citing an FDA warning about the dangers of prescribing the drug to women who are not pregnant, writing, “The FDA said health providers prescribing abortion medication to people who aren’t pregnant are acting without its authorization and that the practice is potentially dangerous for patients.”
“The FDA is concerned about the advance prescribing of mifepristone for this use,” the unnamed FDA spokesperson told POLITICO. “Mifepristone is not approved for advance provision of a medical abortion.
U.S. Senator Cindy Hyde-Smith grills FDA Commissioner Robert Califf on abortion pill May 2024
Abortion Pill Label Notes Potential Risks
So-called ‘self-managed’ abortion was a scheme designed by Big Abortion over a decade ago. Live Action News previously documented that the risks of the abortion pill triggered the FDA to place it under the REMS safety system, where it has remained — even under multiple pro-abortion administrations.
Mifepristone’s 2023 label states that 2.9 to 4.6 percent of women who take abortion drugs end up in the emergency room, indicating that abortion pill ER visits could be in the tens of thousands every year. In addition, the FDA’s medication guide acknowledges that as many as seven percent (7%) of women will need surgery after taking mifepristone ‘to stop bleeding’ or to complete the abortion.
Danco and the FDA always understood that emergency rooms would be necessary to treat abortion pill clients, even as the FDA allowed for the expansion of “self-managed” and “mail-order” abortion. Despite this, abortion insiders — some who are conducting clinical trials on the abortion pill and are cited in studies claiming the drug is safe — have suggested that medical personnel at emergency rooms should falsify medical documents to cover up abortion pill complications.
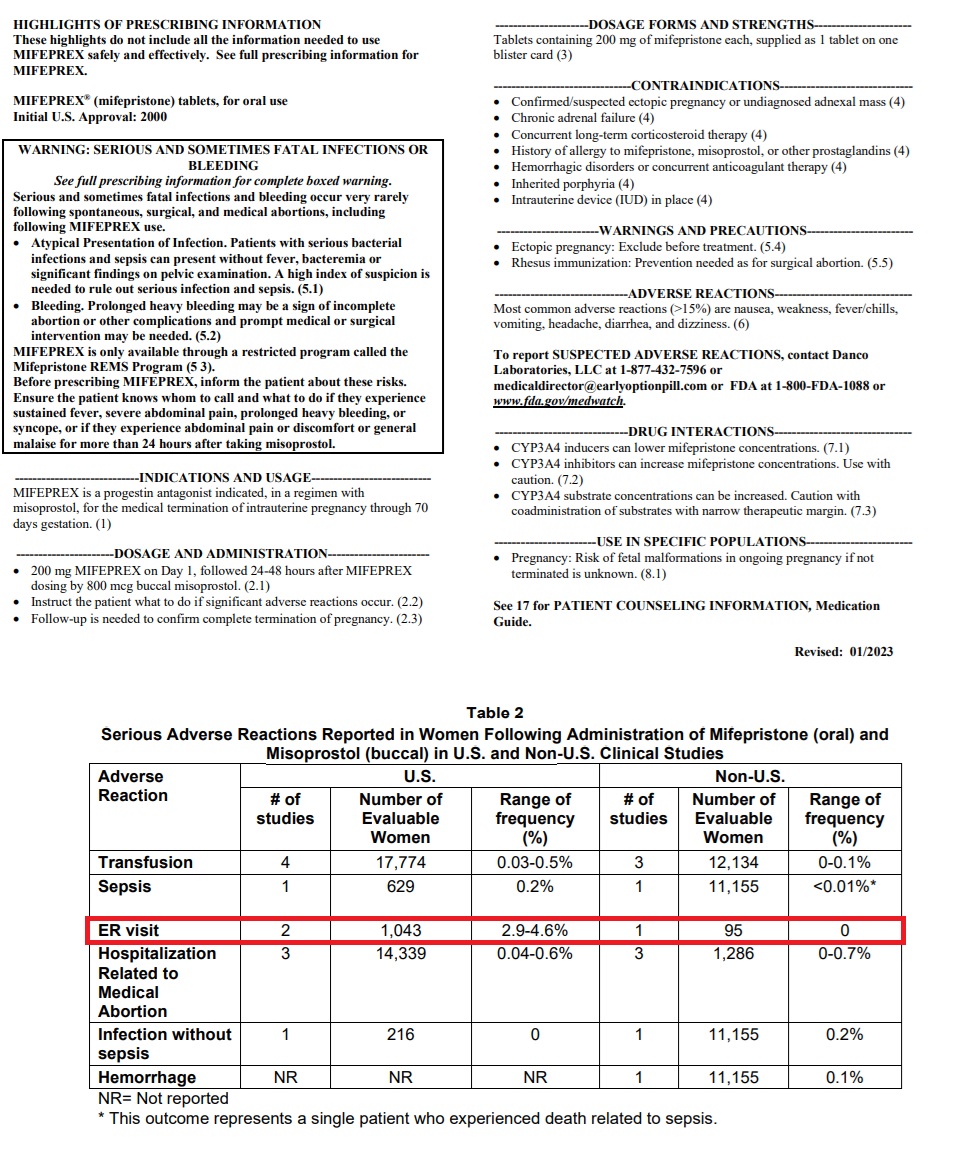
Mifepristone Jan 2023 label shows percentage of women taking abortion pill visit ER
In addition, Live Action News’ “Bad Actors” series has previously documented how multiple bad actors within Big Abortion seem to be in violation of the FDA’s REMS with zero consequences by the FDA. They get away with it, because the FDA has granted policing power to those who profit from the drug (Danco and GenBioPro) who seem to be allowing abortion pill stockpiles to continue.
Mifepristone isn’t FDA-approved for advanced provision
“Is it true that the FDA has not approved Mifepristone for advanced provision of an abortion?” Senator Hyde-Smith asked FDA Commissioner Califf. “When they’re violating the law by allowing them to stockpile what is your responsibility then?”
In response, Califf claimed, “We don’t advocate stockpiling as a method. Again, we don’t regulate the practice of medicine.”
“So, you have no role whatsoever in preventing the stockpiling of this?” the Senator asked.
“I don’t know how we would,” Califf claimed.
But the FDA could pressure Danco and GenBioPro to properly police their prescribers and decertify anyone who violates the FDA’s REMS, which Califf failed to mention.
“It’s completely unacceptable that the FDA could just open the floodgates to these chemical abortion pills and not accept any responsibility when that access is abused through the advance prescribing of mifepristone,” said Hyde-Smith (emphasis added). “Simply saying the FDA does not ‘regulate the practice of medicine’ should not absolve the FDA from the ramifications of the women and girls who could be harmed by these abortion drugs.”






