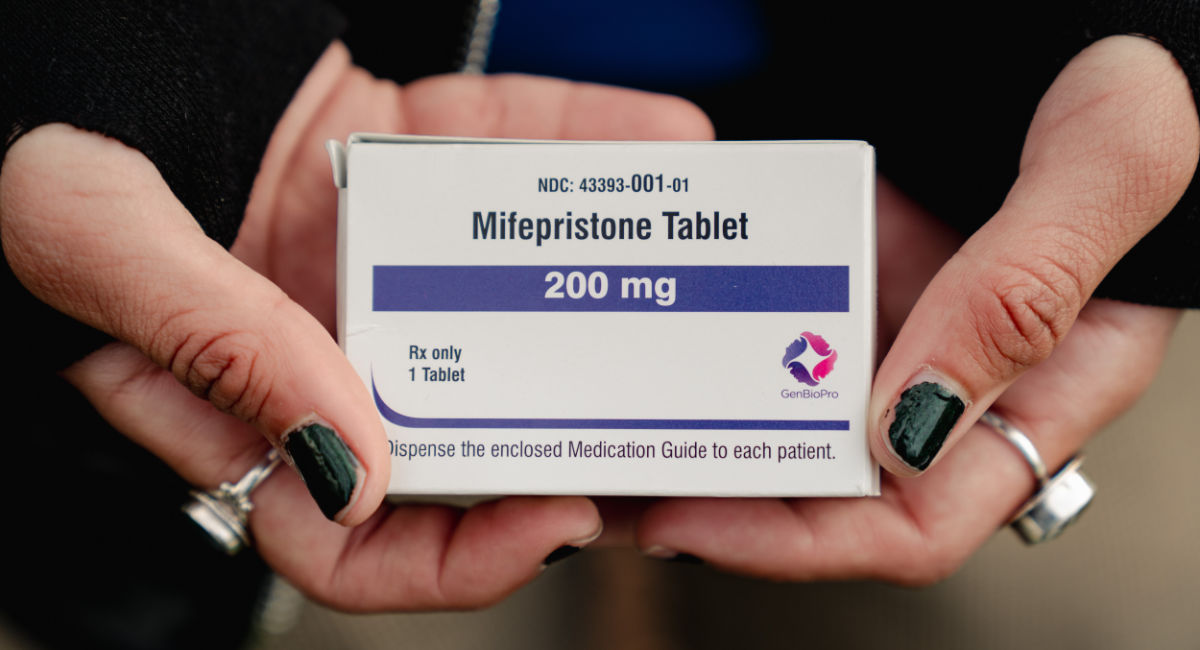
The U.S. generic abortion pill manufacturer controlling roughly two-thirds of the abortion pill market has filed a motion to intervene in a lawsuit that is challenging the expansion of the abortion pill. The company says the outcome of the case could cause “serious harm” to its “economic interests.”
GenBioPro, according to a court document, is the “sole supplier of generic mifepristone in the United States,” and its sales of generic mifepristone and misoprostol (the abortion pill regimen) “represent the majority of the company’s revenue.”
The name brand manufacturer of the abortion pill, Danco, is a competitor to GenBioPro, and has its own interests to protect in the lawsuit, Missouri et al. v. FDA, filed by the State of Missouri, State of Kansas, and the State of Idaho in October 2024 in the U.S. District Court for the Northern District of Texas, Amarillo Division.
The States claim that their “sovereign” interest in “protecting… citizens” was violated by the U.S. Food and Drug Administration’s (FDA) decision to allow the abortion pill to be dispensed via mail. These states chose to intervene and file a complaint after the U.S. Supreme Court ruled that the original plaintiffs (a group of pro-life physicians) in the case [formerly known as Alliance for Hippocratic Medicine (AHM) v. FDA], had no standing to sue.
GenBioPro’s Motion to Intervene
As GenBioPro (GBP) claims in the Court document, the States are seeking an “order rescinding FDA’s 2019 approval of GenBioPro’s generic mifepristone as safe and effective for its intended use. The States also ask the Court to vacate other regulatory actions that impact the conditions under which mifepristone can be prescribed and distributed to patients, threatening serious harm to GenBioPro and the many Americans it serves,” because the sale of the abortion pill regimen “comprises the majority of GenBioPro’s business.”
“FDA is GenBioPro’s regulator, and Danco is GenBioPro’s competitor,” GBP added, noting that it filed the motion once it became “aware that its interests would no longer be protected” by Danco, which was the “original defendant.”
GBP claimed that, since the original AHM lawsuit, “the nature of the threat to GenBioPro’s interests has changed dramatically,” as “the Amended Complaint discusses GenBioPro more than 25 times.” In short, the scope of the lawsuit’s complaint has changed considerably.
While “Danco’s interests and GenBioPro’s interests were initially aligned when the Alliance Plaintiffs challenged… the Amended Complaint” by the three States “now alleges that the 2019 ANDA approval [of the generic abortion pill] was ultra vires and arbitrary and capricious under the Administrative Procedure Act (‘APA’) in its own right,” GBP noted, “separately targeting GenBioPro’s 2019 ANDA approval on grounds unrelated to the 2000 Mifeprex approval.”
GBP wrote that “… Danco’s interest in this action now—to protect post-2000 regulatory decisions addressing distribution of Mifeprex—does not necessarily extend to protecting the approval of generic mifepristone produced by its competitor,” and it is now “obvious that the economic interests of [GenBioPro] are at stake.”
Since this lawsuit would impact “GenBioPro’s ability to market its product in the United States,” it is “a direct, concrete, and particularized threat to GenBioPro’s economic interests that justifies intervention,” GBP claimed. “If the States’ claims succeed and the challenged FDA decisions on mifepristone are vacated, GenBioPro may be hindered in marketing its primary product and face severe financial and operational distress.”
GenBioPro is represented in the lawsuit by a team of lawyers from the Democracy Forward Foundation (DFF) and Arnold & Porter, the same team which represented the abortion pill company in the AHM case. DFF partners with multiple pro-abortion groups such as Advancing New Standards in Reproductive Health (ANSIRH), the American College of Obstetricians and Gynecologists (ACOG), and Planned Parenthood. In past years, Warren Buffett’s abortion philanthropy via the Buffett Foundation (which invested in the abortion pill early on) has granted over $4 million to DFF.
“If the judge grants GenBioPro’s request, the maneuver will allow the company to lead the defense of mifepristone,” the New York Times claimed.
GenBioPro Holds Majority of Abortion Pill Market
A 2023 GenBioPro lawsuit claimed that the company sold only “generic mifepristone and misoprostol, which make up the FDA-approved two-drug regimen for medication abortion.” Located in Las Vegas, Nevada, GBP has claimed to ship a significant amount of the life-ending abortion drugs across the nation. The company previously claimed that sales from the abortion pill regimen are “essentially the company’s sole source of product revenue” and that it had “captured a significant share of the nationwide market for mifepristone.”
According to multiple media sources, GBP’s share is massive, supplying roughly two-thirds of U.S. abortion pills.
“Since 2019, when GenBioPro received approval from FDA to sell generic mifepristone, GenBioPro has marketed and sold more than 850,000 units of the product throughout the United States,” the company wrote in 2023. “Between 2017 and 2020 (the year after GenBioPro began marketing its product), medication abortions in the United States increased by 45 percent, even as the overall number of abortions has declined since the 1990s. Medication abortion now accounts for the majority of pregnancy terminations in the United States.”
GenBioPro only manufacturers one product: the generic abortion pill regimen
Abortion Pill Expanded Quickly During Pandemic
The abortion pill (mifepristone 200mg) was approved by the FDA in 2000 for use in the termination of pregnancy with the drug misoprostol. Mifeprex was originally manufactured by Danco Laboratories, but in 2019, the FDA approved GenBioPro as mifepristone’s generic manufacturer.
Under a safety system called REMS, the drug was only permitted to be dispensed in-person by a certified prescriber (doctor or clinician). But in April of 2021, under the guise of the COVID-19 pandemic, the Biden administration FDA expanded the REMS and temporarily enabled the limited mail-order pharmacy distribution of mifepristone (the abortion pill).
In December 2021, the FDA further weakened the REMS by eliminating the in-person dispensing requirement and enabling the abortion pill to be permanently shipped by mail.
In January 2023, the Biden FDA announced it would also allow retail pharmacies to dispense the drug.
GenBioPro Funding
GenBioPro’s president, Evan Masingill, was one of the first board members to join the abortion facility Desert Star Institute for Family Planning (DSIFFP) in 2017. That clinic was founded by DeShawn Taylor, one Planned Parenthood’s former abortionists recorded in undercover video by the Center for Medical Progress admitting to committing abortions “up to 24 weeks” and needing to “hit the gym” to do D&Es without first causing fetal death using digoxin. Taylor is now a senior medical director and consultant for GenBioPro, and claimed in a Dobbs v. Jackson case Supreme Court brief that “abortion saves lives.”
GenBioPro has been funded by the pro-abortion David and Lucile Packard Foundation, just like the originally approved and highly secretive abortion pill manufacturer Danco Laboratories (which received $14.2 million in startup investment dollars from Packard). According to a recent IRS document obtained by Live Action News, the Packard Foundation has invested millions in GenBioPro:
- $150K for “Just Societies” in 2023
- $1.5M/2019 to “increase reproductive health access”
- $500K/ 2018 to “increase reproductive health access”
- $100K/2017 for “the development, submission and executing of a pre-investigational new drug”
- $185K/2016 for “increasing access to reproductive health services”
- $2 million/2015, according to a recent IRS document (below)
GenBioPro is also listed under Packard’s “Other Asset Schedule” for 2019 and 2018.
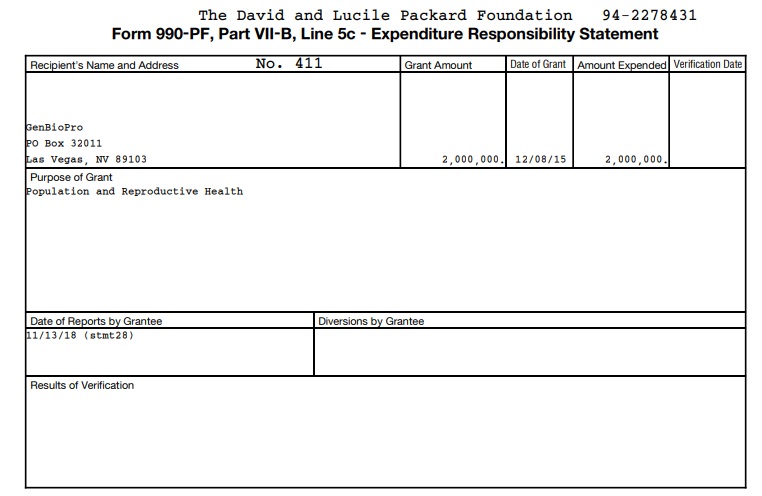
Packard Foundation funds generic abortion pill GenBioPro – $2 million in 2015
In recent years, the Marguerite Casey Foundation (MCF), an an invitation-only grantmaking organization led by Dr. Carmen Rojas, granted GBP $800,000 towards “clinical trials” to establish FDA-approved indications for mifepristone. MCF “holds shares in the healthcare industry including shares in Tenet Healthcare Corporation, which owns facilities in 19 states where abortion is restricted,” the Foundation’s LinkedIn page claims.
In addition, the Soros Economic Development Fund “invested” $3 million in GBP, and in 2023, the Cumming Foundation “invested” $1 million in the abortion pill manufacturer “to increase the availability of low-cost medical care.”
In 2020, the San Francisco-based Parsemus Foundation “invested” $1.4 million in GBP and has been granting thousands in loan dollars ($500K in 2019, $275K in 2018 and $320K in 2017) for so-called “operations related to Family Planning.” The Parsemus Foundation was founded by Elaine Lissner in 2005 “to support further research on male contraceptives,” the foundation’s website states.
Data recently published by the FDA reveals abortion pill usage has increased nearly 103% since 2018, the year just prior to GenBioPro’s approval for the generic dug. Tragically, as of December 2024, the FDA has estimated approximately 7.5 million women “have used mifepristone in the U.S. for medical termination of pregnancy” since 2000 when the drug was initially approved.