Danco Laboratories, the company in charge of the manufacturing and distribution of the abortion pill in the United States, must now comply with federal law by publishing the manufacturing country of origin on its packaging.
March 2025 packaging information uploaded to the U.S. Food and Drug Administration (FDA) Report, Daily Med, MedLibrary.org, and the FDA’s Label Search website reveals that Danco is currently importing the abortion pill from Spain. Past reports showed the pills came out of China.

Danco label (3/25) of abortion pill mifepristone 200mg MFG in Spain
What is the Law
“U.S. law provides that all products of foreign origin imported into the United States must be marked with their country of origin. This requirement protects consumers’ interests by informing the ultimate purchaser as to the country of origin of the goods the U.S. consumer is purchasing, including prescribed medications. This fact sheet discusses the country of origin marking requirements applicable to prescription medications of foreign origin imported into the United States for retail sale,” an August 2024 U.S. Customs and Border Protection document states.
“The Tariff Act of 1930 requires companies that import foreign products into the United States to mark the country of origin on those products. Importers that fail to mark their products are subject to a 10% ad valorem duty,” claimed the Department of Justice (DOJ) in its 2023 False Claims Act settlement against Danco.
U.S. Customs and Border Protection (CPB) New York Field Office Director Frank Russo stated, “The United States has required imported goods to be marked with their country of origin for generations, so American consumers can use that information in their purchasing decisions.”

US Customs on origin of country for drug labeling
Who is Danco Laboratories?
In 1994, with the encouragement of the Clinton administration, Roussel-Uclaf assigned the U.S. rights of marketing and distribution of the abortion pill (known then as RU-486) to the eugenics proponents at the Population Council. The right to distribute the drugs was later handed over to Danco Laboratories, a sub-licensee of the Population Council. By 1996, the Population Council (funded in part with investments from the Buffett and Packard Foundations) had submitted its application for the drug to the FDA, and a series of clinical trials began.
In 2000, following those clinical trials, the U.S. FDA approved mifepristone as an abortion pill for use up to seven weeks of pregnancy in a regimen along with the drug misoprostol. In 2019, the FDA approved GenBioPro to become the generic manufacturer of mifepristone (brand name Mifeprex).
Initially, as Live Action News previously documented, Danco itself was kept secret, and according to a 2000 Washington Post article, the FDA “took the unprecedented step of refusing to disclose the name or location of the manufacturer.” In a separate article, the Washington Post wrote, “Secretive and obscure, Danco is one of the most enigmatic companies in the pharmaceutical industry… They say Danco intends to make a profit eventually.”
Danco remained an elusive and highly secretive company, and for more than two decades, its manufacturing location sites have been undisclosed, as have the company’s executives, investors, and office locations.
Follow the money
In 2023, a Department Of Justice (DOJ) False Claims Act settlement fined Danco just under $800,000 for allegedly illegally withholding information about Mifeprex’s manufactured country of origin — which, according to Life Legal Defense Foundation’s release, was China at that time.
The settlement was signed by W. Bradley Daniel on behalf of Danco.
This is significant because up to this time the abortion pill manufacturer did not include the country of origin on Mifeprex packaging.

Danco False Claim Act settlement signed by W Bradley Daniel
Around that same time, a report published by Mother Jones (MJ) unveiled a January 2023 court document, which appears to describe a shareholder power struggle happening within Danco. Both the DOJ Settlement as well as MJ’s piece have identified W. Bradley Daniel as a leading Danco investor, who MJ claimed “earned about $10.3 million in fees alone.”
This has caused some to speculate whether a petition filed by Danco to the U.S. Supreme Court in the Alliance for Hippocratic Medicine (AHM) abortion pill lawsuit challenging the expansions of the drug under the FDA’s safety system known as REMS, was less about “protecting women” and more about the bottom line for Danco’s investors.
According to MJ, the “average return on investment for everyone who invested in Danco was about 452 percent over 23 years.”
In June of 2024, the U.S. Supreme Court ruled that the plaintiff doctors in the AHM lawsuit did not have standing to sue. However, in October of 2024, U.S. District Court Judge Matthew J. Kacsmaryk ruled that three states (who likely have standing in the case) could intervene.
Danco’s Abortion Pill Manufacturing Location Surfaces
The FDA does not publish the locations of manufacturing plants. Live Action News reached out to inquire about the location, but was told by an FDA spokesperson, “Consistent with our obligations under applicable laws, including the Trade Secrets Act, the FDA does not disclose information submitted by the Applicants to FDA about manufacturing sites for Mifeprex and the generic for Mifeprex, Mifepristone Tablets, 200 mg.”
However, the FDA acknowledged that it “conducts inspections and assessments of FDA regulated drug manufacturing facilities to determine a firm’s compliance with applicable laws and regulations.”
READ: DEFUND Planned Parenthood: A disturbing record of abortion coercion
Despite the consistent secrecy of the drug maker, Live Action News previously documented that in 2000, Chinese officials confirmed that RU-486 was being manufactured in China by the Shanghai-based Hua Lian Pharmaceutical Co. — owned by the Chinese Communist Party.
The Los Angeles Times also confirmed the location, writing, “Danco refuses to release the names of its executives and investors. The company even persuaded the FDA to keep secret the location of the factory where the abortion drug will be produced, despite several published reports that it will be made in bulk at the state-owned Hua Lian Pharmaceutical Co. in Shanghai.”
In 2008, Hua Lian distributed contaminated leukemia drugs that paralyzed at least 200 Chinese cancer patients, according to a New York Times report. The contaminated drug problem was such a concern that even abortion corporation Planned Parenthood called on FDA officials to investigate, writing, “The New York Times reported last week that Shanghai Pharmaceutical Group, and its subdivision, Shanghai Hualian, are under investigation for producing tainted leukemia drugs in China. Reports indicate that these drugs were not imported into the United States and that no other drugs produced by the company have been found to be faulty. Among the many drugs produced by Shanghai Hualian is mifepristone, one of two drugs used in medication abortion.”
Then, in 2022, ABC News confirmed with a Danco spokesperson that the deadly abortion drug was at that time no longer being manufactured in China, but in Europe. By the spring of 2025, the company was publicly showing Spain as the country of origin on its packaging.
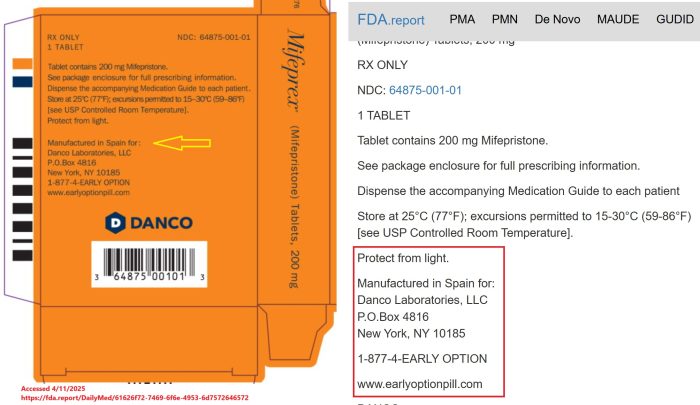
Danco Packaging shows abortion pill country of origin is Spain March 2025
Generic Abortion Pill Maker
While Danco was hit with a False Claims Act settlement, labeling for GenBioPro (GBP) does not appear to show a country of origin, according to the latest packaging at the Daily Med or MedLibrary.org.
A May 2020 import report appears to indicate that at one time GBP received mifepristone tablets from Sun Pharmaceuticals Industries, LTD. The bill of lading import record shows the abortion drugs were shipped from Sun Pharmaceuticals’ Mumbai location after departing from a port in Nhava Sheva, India. The drugs were shipped to GenBioPro’s Nevada office at 3651 Lindell Rd, STE D1041 in Las Vegas after landing at a port in the New York/Newark Area of Newark, New Jersey.
But, while it is unclear whether GBP is still importing, drug packaging under the GBP name, including a 2024 packaging label for misoprostol and a March 2023 mifepristone label located in the FDA Report as well as the FDA label search website, fails to show the country of origin.
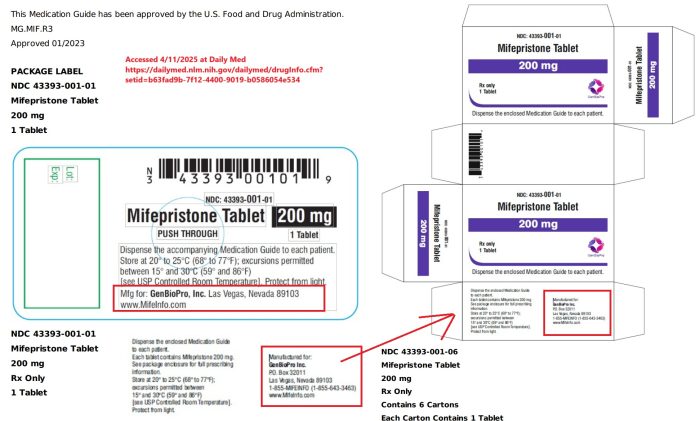
GenBioPro Label Packaging from Daily Med as of Jan 2023
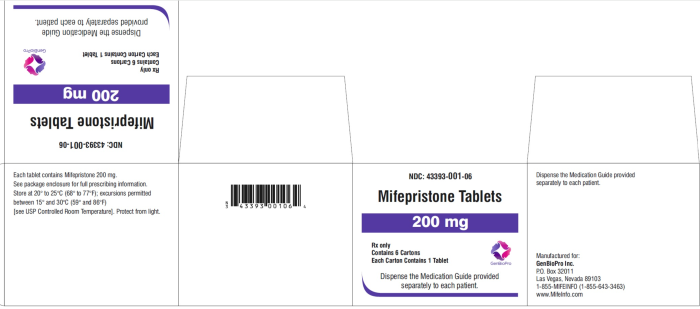
GenBioPro abortion pill generic FDA packaging as of 2023
In 2023 alone, the Guttmacher Institute (Planned Parenthood’s former “special affiliate” and research arm) estimated that the abortion pill accounted for a total of 648,500 abortions.
In February 2025, the FDA issued a report noting the likely millions of preborn lives now ended by the abortion pill, claiming that since 2000 when the abortion pill was approved, the “number of women who have used mifepristone in the U.S. for medical termination of pregnancy through the end of December 2024” has now climbed to “approximately 7.5 million…”
It is clear that abortion pill companies are self-policing, when customs and the FDA should be scrutinizing their actions to hold them to account.
Despite legal challenges and the FDA’s continued secrecy around the drug, mail-order abortion pills could be stopped immediately if the Federal Comstock Act — which essentially prohibits the mailing of “any article, instrument, substance, drug, medicine, or thing [that] may, or can, be used or applied for producing abortion” — were to be enforced by the Trump Administration.