How does the abortion pill affect a preborn baby in the event that a woman chooses to attempt to reverse the effects of the pill? Abortion advocates claim that not completing the abortion pill process and attempting to continue the pregnancy means the child could have fetal abnormalities. The abortion industry has used this scare tactic to pressure women to continue the abortion pill process, a regimen that includes two different medications (mifepristone and misoprostol).
Planned Parenthood, which profits from abortion and holds the largest market share of the abortion industry with plans to expand abortion, tells women online, “Some of the medicines used in medication abortion may cause serious birth defects if the pregnancy continues. So, in the unlikely case that it doesn’t work, you will need to have an aspiration abortion to end the pregnancy.” This claim is reiterated in additional online publications. What Planned Parenthood isn’t telling women is that taking the first pill, mifepristone (a progesterone blocker), actually comes with no risk of birth defects, should they change their minds and seek abortion pill reversal treatments (which attempt to counteract mifepristone’s effects by administering progesterone).
READ: Four lies abortion supporters are telling about abortion pill reversal… and the truth
In part one of this two part series, Live Action News documented that during original clinical trials of the abortion pill, there was little data to conclude that a continued pregnancy after use of the abortion pill would lead to birth defects, especially when the woman only took the first pill of the two pill regimen. Years later, as part two will demonstrate, even more data confirms the same: the abortion industry is lying to women by claiming that not completing the abortion pill process raises their risk of fetal abnormalities.
First, a little history of the abortion pill…
From the Congressional Research Service:
- January 22, 1993: President Bill Clinton directed Department of Health and Human Services (HHS) to “assess initiatives” for the promotion of testing, licensing, and manufacturing RU-486 (abortion pill).
- April 1993: The Population Council and French pharmaceutical company Roussel Uclaf announced a preliminary agreement whereby the company would license rights for the drug’s production to the Population Council.
- May 16, 1994: The Clinton Administration announced that Roussel Uclaf would donate U.S. patent rights to the Population Council; the right to distribute the drugs were later handed over to Danco Laboratories, a sub-licensee of the Population Council.
- October 1994 to September 1995: The Population Council conducted clinical trials of RU-486.
- September 28, 2000: The FDA approved the abortion pill regimen — mifepristone along with misoprostol — for the termination of early pregnancy.
Next, the studies…
2000: The abortion pill’s (Mifeprex) 2000 FDA label reads, “As of May 2000 a total of 82 cases have been reported in which women with on-going pregnancies after using mifepristone alone or mifepristone followed by misoprostol declined to have a surgical procedure at that time.” The results, seen in the image below, revealed few cases of abnormalities were detected:
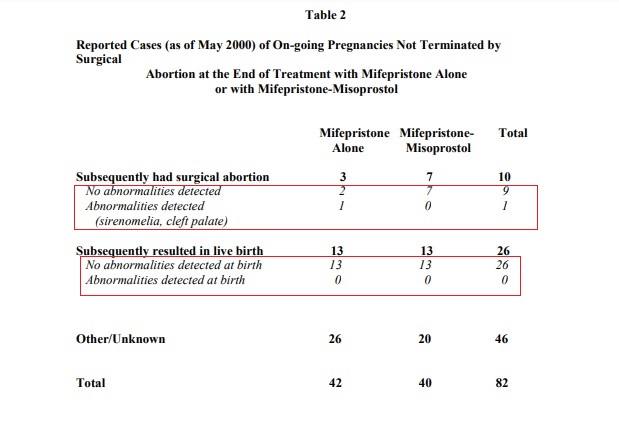
Mifeprex 2000 FDA label continued pregnancy fetal deformity risks
Animal studies mentioned in the 2000 label noted that, “deformities were most likely due to the mechanical effects of uterine contractions resulting from decreased progesterone levels.”
Summaries from Phase IV studies published by the FDA reported that, out of 82 cases of ongoing pregnancies reported in Europe, there were no reports of birth defects among 26 women who carried their pregnancies to a live birth.

FDA Phase 1V few birth defects in ongoing pregnancies: FDA Abortion Pill Study
2001: Congressional Research Service
A February 2001 Congressional Research Service report quoted a Lancet study of 71 instances where mothers changed their minds after starting medication abortions, and concluded that few of those continued pregnancies resulted in fetal malformations:
- 1 occurred with mifepristone alone.
- 7 with prostaglandin gemeprost.
- 0 were associated with use of mifepristone and misoprostol.

CRS footnote few birth defects from abortion pill in ongoing pregnancies
2006: FDA Director Janet Woodcock
Janet Woodcock was the director of the FDA’s Center for Drug Evaluation and Research (CDER) when the agency approved the abortion pill. In May of 2006, as the FDA’s M.D. Deputy Commissioner for Operations, Woodcock spoke before the Subcommittee on Criminal Justice, Drug Policy and Human Resources House Committee on Oversight and Government Reform, where she stated:
Danco Laboratories, the sponsor of the Mifeprex NDA, is currently conducting the pregnancy follow-up study; it has designed and implemented a protocol, but it has determined that as of this time very few pregnancies (less than 20) were continued. For the number that were continued, Danco informs the Agency it has been unable to collect outcome data on any of them because of difficulties enrolling patients…
2013: BJOG (British Journal of Obstetrics and Gynecology) study
A study titled, Continuation of pregnancy after first-trimester exposure to mifepristone: an observational prospective study, and published January of 2013 looked at whether pregnancies continued after the first or both pills in the abortion pill regimen carried an excess risk for fetal malformations. According to Journal Watch, “Between 1997 and 2010, French investigators studied the outcomes of 46 pregnancies that continued after exposure to mifepristone alone and 59 after exposure to both mifepristone and misoprostol before 13 weeks’ gestation.”
They found that the overall rate of malformations was “only slightly higher than that expected in the general population.”
The authors investigated each case thoroughly and wrote that, “Taken together, the six cases of major or minor malformations occurring in patients exposed to mifepristone do not suggest a particular pattern of malformations that may be related to this drug.”

BJOG Mifeprex abortion pill malformation study 2013
2016: FDA Mifeprex Label
Updated labeling from the FDA in 2016 found that when women took both pills, Mifepristone combined with Misoprostol, “Birth defects have been reported…” But the label also states that, “The risk of adverse developmental outcomes with a continued pregnancy after a failed pregnancy termination with MIFEPREX in a regimen with misoprostol is unknown.”
In addition, the FDA’s medication guide states, “The chance of birth defects if the pregnancy is not ended is unknown.”

FDA 2016 Mifeprex Medication Guide birth defects unknown in abortion pill
The “unknown” factor is included in FDA’s most recent April 2019 label as well.
Women who choose to reverse the effects of the abortion pill need facts, not scare tactics that always manage to result in the death of the preborn child.
Read Part One of this series here.
Editor’s Note, 7/30/24: This article has been updated to remove references and links to a study that is no longer published at the designated links.
“Like” Live Action News on Facebook for more pro-life news and commentary!