
Missouri's Prison Nursery Program 'changes lives' of moms and their babies
Bridget Sielicki
·
Abortion Pill·By Nancy Flanders
Could next year’s budget bill eliminate the mail-order abortion pill?
Last Wednesday, the House Appropriations Committee advanced a budget bill in relation to the Food and Drug Administration (FDA). The bill, which passed the committee 34-27, includes a provision that would disallow the mail-order abortion pill.
In December of 2021, the Biden administration FDA decided to eliminate the in-person dispensing requirement in place on the abortion pill since 2011. This decision increased access to mifepristone (the first drug in the abortion pill regimen) by allowing it to be prescribed via telehealth appointments and distributed through the mail.
The bill states, “The modifications made by the Food and Drug Administration on January 3, 2023 to the risk evaluation and mitgation strategy [REMS] under section 505-1 of the Federal Food, Drug, and Cosmetic Act … for mifepristone are hereby nullified.”
Pro-abortion Rep. Norma Torres introduced an amendment to remove the mifepristone provision from the bill but that amendment was defeated in a voice vote.
The REMS guidelines were placed on the abortion pill more than a decade ago due to the serious risks that are associated with mifepristone. The abortion pill has been found to be four times more dangerous for women than a first-trimester surgical abortion. Complications include incomplete abortion, hemorrhaging, and infection that can cause death.

The results of a Gynuity-sponsored study published in 2021 in the journal Contraception found that six percent (6%) of 1,157 women who took the abortion pill visited the emergency room or an urgent care center for abortion pill-related complications. That data is reflective of abortion pill complication rates in the United Kingdom.
By removing the REMS guidelines, the FDA put women at risk of hemorrhaging and death if they take the abortion pill while experiencing an undiagnosed ectopic pregnancy. In addition, the further along a woman is in her pregnancy, the more ineffective the abortion pill becomes, as it is only currently allowed to be used up to 10 weeks of pregnancy (originally seven weeks at the time of FDA approval in 2000). Women who take the abortion pill after 10 weeks are at a higher risk of incomplete abortion which can lead to infection.
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Bridget Sielicki
·
Abortion Pill
Angeline Tan
·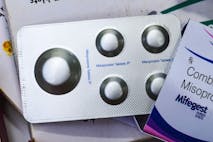
Abortion Pill
Nancy Flanders
·
Analysis
Cassy Cooke
·
Politics
Bridget Sielicki
·
Human Interest
Andrea Trudden
·
International
Nancy Flanders
·
Politics
Nancy Flanders
·
Pop Culture
Nancy Flanders
·
Politics
Nancy Flanders
·
Politics
Nancy Flanders
·