
Details shrouded in secrecy as third woman in a month is injured at Rhode Island Planned Parenthood
Bridget Sielicki
·
Abortion Pill·By Carole Novielli
What you should know about the dangerous ‘self-managed’ abortion pill push
Live Action News has been monitoring potential changes in the way the abortion pill could be dispensed in the future, and there is cause for immediate concern. The changes, should the Food and Drug Administration (FDA) allow them, could mean that the drugs would become accessible to anyone via mail order, pharmacy distribution, and possibly online. Not only would this change expand early abortions, but it could also dramatically affect what limited tracking of abortion statistics and complications/deaths are reported.
Currently, under FDA’s approved safety system, REMS (Risk Evaluation and Mitigation Strategy), the abortion pill can only be ordered, prescribed, and dispensed by — or under the supervision of — a hospital or clinic healthcare provider who meets certain qualifications, such as the ability to:
Assess the duration of pregnancy accurately.
Diagnose ectopic pregnancies.
Provide surgical intervention in cases of incomplete abortion or severe bleeding.
The abortion pill regimen, which consists of the drugs Mifeprex and Misoprostol, was approved by the FDA on September 28, 2000, for up to 49 days (7 weeks) of pregnancy. In 2016, the FDA approved the pill for an extended time frame of up to 70 days, or 10 weeks.
By the first half of 2001, according to Guttmacher, early medication abortions accounted for just six percent of procedures. By 2014, according to Guttmacher, early medication abortions accounted for 31% of all nonhospital abortions and 45% of abortions before nine weeks’ gestation. The former “special affiliate” of Planned Parenthood estimated 272,400 early medication abortions were provided in nonhospital facilities in 2014. According to the FDA, between 2000 and the end of 2017, an estimated 3.4 million women had used the abortion pill.
Promoting ‘Self-Managed’ Abortion
The abortion lobby is attempting to expand access to the abortion pills via mail order or pharmacy by pushing “self-managed abortion,” described by Guttmacher as ending a pregnancy “w/o direct supervision by a health care provider.” To accomplish this, REMS, which, according to the FDA is “a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use,” must be eliminated.
To that end, the abortion industry is conducting clinical trials, due to be completed very soon.
1). Direct-To-Consumer (pills sent via mail after TelAbortion or Telemedicine interview)
“After consulting with an abortion provider by videoconference, qualifying participants are sent the necessary abortion medicines by mail.”
Patient does not have to travel to abortion facility
Abortion provider conducts video evaluation over Internet
Tests done at medical facilities near patient’s home
Abortion pills then sent by mail
Planned Parenthood taking part in study
Sponsored by Gynuity Health Projects
Gynuity’s principal investigator, Dr. Elizabeth Raymond, told the New York Times that if the study shows telemedicine and mail approach works, it could encourage the FDA to stop restrictions on mifepristone.
Due to complete June 2019
“Women participating in this study will obtain mifepristone and misoprostol from the pharmacy instead of in the clinic.”
Could result in abortion pills by prescription at universities and schools as well as numerous medical offices
Sponsored by University of California San Francisco with abortionist/researcher Dr. Daniel Grossman as contact
Grossman authored study of OBGYN attitudes on abortion published in Journal Obstetrics & Gynecology, and claimed that “among OB-GYNs whose patients have asked for an abortion but who do not now offer it, 28 percent said they would offer the drugs if the FDA lifted its most onerous restrictions on their use.”
Due to complete July 2019
For participants who are “not currently pregnant and not desiring to be pregnant in the next year.”
Live Action News previously detailed this study.
Sponsored by the University of California, San Francisco (UCSF)
Contact includes (again) abortionist Daniel Grossman
Due to complete April 2020
In addition to the clinical trials, the ACLU is filing lawsuits to attempt to force the FDA to remove Mifeprex from REMS.
Article continues below
Dear Reader,
In 2026, Live Action is heading straight where the battle is fiercest: college campuses.
We have a bold initiative to establish 100 Live Action campus chapters within the next year, and your partnership will make it a success!
Your support today will help train and equip young leaders, bring Live Action’s educational content into academic environments, host on-campus events and debates, and empower students to challenge the pro-abortion status quo with truth and compassion.
Invest in pro-life grassroots outreach and cultural formation with your DOUBLED year-end gift!
Although abortion advocates will claim that the abortion pill is safe, a 2015 study of abortion safety in California — based on comprehensive and reliable data from Medicaid billing records rather than surveys — found that medication abortions resulted in four times the complication rate of first-trimester surgical abortions, according to a review of the data published by National Review Online. Professor Michael New and Donna Harrison, MD, write, “Given that chemical abortions are already riskier than early surgical abortions, it stands to reason that performing medical abortions without physician supervision only increases those risks.”
In 2018, NPR spoke with Dr. Donna Harrison, a board-certified obstetrician and gynecologist, and executive director of the American Association of Pro-life Obstetricians and Gynecologists (AAPLOG), who noted, “[D]ata on complications from abortion is incomplete because doctors and patients don’t always report it accurately, or at all.”
“There is no good data collection — and when you have garbage in, and you have garbage out,” Dr. Harrison stated.
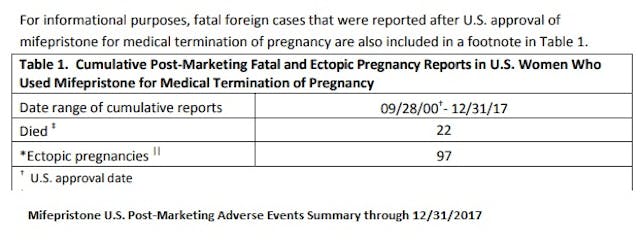
Currently, according to the FDA, as of December 31, 2017, there have been reports of 22 deaths associated with the abortion pill regimen and several cases of severe systemic infection (also called sepsis), including some that were fatal.
READ: Cecile Richards claims abortion pill ‘safer than Tylenol.’ She’s very wrong.
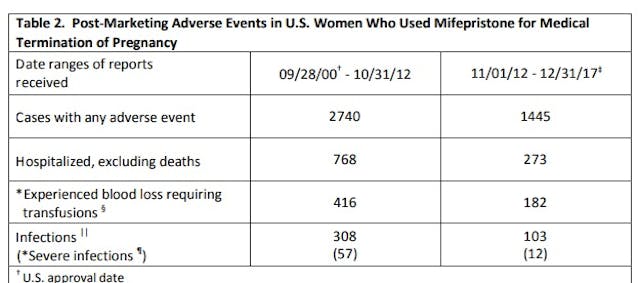
In addition to deaths, between 2000 and 2012, numerous serious complications were also reported:
Cases with any adverse event – 2740 (average 228/yr)
Hospitalized, excluding deaths – 768 (average 64/yr)
Experienced blood loss requiring transfusions – 416 (average 35/yr)
Infections – 308 (average 26/yr)
Within the past five years (between 2012 and 2017) complication reports from the abortion pill have remained steady:
Cases with any adverse event – 1445 (average 289/yr)
Hospitalized, excluding deaths – 273 (average 55/yr)
Experienced blood loss requiring transfusions – 182 (average 36/yr)
Infections – 103 (average 21/yr)
In one state alone — Ohio — complications from the abortion pill have increased an astronomical 87 percent since 2014.
Planned Parenthood and the abortion industry present abortion as “safe and simple,” but one look at the forms they require women to sign reveals the real truth. Missed abortion, blood clots, infection and even death are among the risks associated with medication abortion.

In part two, Live Action News will further detail conflicts within organizations pushing these changes — specifically the funding of a large investor of the pill’s manufacturer.
“Like” Live Action News on Facebook for more pro-life news and commentary!
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Bridget Sielicki
·
Human Interest
Andrea Trudden
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Bridget Sielicki
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·