
Lithuania launches initiative to care for premature and sick infants
Angeline Tan
·
Abortion Pill·By Carole Novielli
The abortion industry wants the abortion pill approved for miscarriage care… but why?
The abortion industry and its media allies have been busy spreading disinformation by conflating miscarriage and abortion, early delivery and abortion, ectopic pregnancy treatment and abortion, and more.
“A miscarriage — the completely unintentional death of a child in-utero — is not remotely related to an actual abortion. Actual abortion — whether conducted through means of the abortion pill or surgical procedures — is the on-purpose, consciously chosen, and fully intended violent taking of a child’s life. The distinction between miscarriage and abortion becomes clear when Dr. Anthony Levatino’s videos describing abortion procedures are viewed,” explained Live Action News contributor Kristi Burton Brown.

The U.S. Food and Drug Administration (FDA) approved the abortion pill regimen (mifepristone 200mg — which starves the preborn child of nutrients — in 2000, along with misoprostol, a second drug which causes contractions to expel the baby). It was later placed under an FDA safety system known as REMS, after multiple deaths prompted closer scrutiny of the drug. The REMS limits who can prescribe or dispense mifepristone 200mg; not all medical professionals have access to the abortion pill.
Do calls to remove or limit use of the abortion pill regimen negatively impact miscarriage care, as the media is attempting to claim?
ACOG promotes abortion pill regimen for miscarriage
The American College of Obstetricians and Gynecologists (ACOG), which heavily promotes abortion and typically changes its recommendations to support the talking points of the abortion lobby, is openly promoting use of both drugs in the abortion pill regimen for so-called “early pregnancy loss” — otherwise known as miscarriage care.
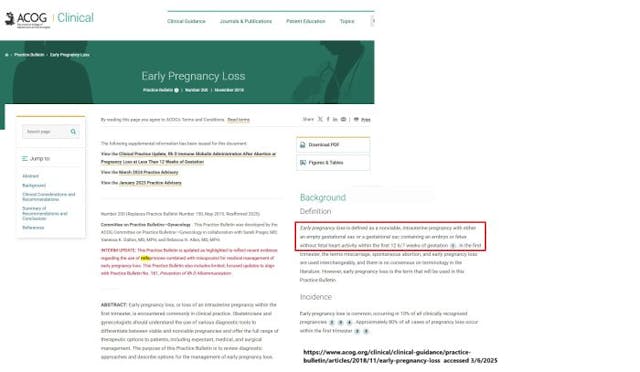
“Early pregnancy loss is defined as a nonviable, intrauterine pregnancy with either an empty gestational sac or a gestational sac containing an embryo or fetus without fetal heart activity within the first 12 6/7 weeks of gestation,” ACOG wrote in its Practice Bulletin, Number 200 (replaces Practice Bulletin Number 150, May 2015. Reaffirmed 2025).
Ironically, here the ACOG appears to suggest that a living “embryo or fetus” has “fetal heart activity,” despite working tirelessly to influence the positions of its members regarding abortion. It has regularly changed its own long-held messaging and definitions on when life begins (fertilization), prenatal heartbeat, and other developmental markers.
Despite the fact that there is a stark difference between abortion — which intentionally takes the life of a living preborn baby — and miscarriage care (the treatment after the preborn baby has naturally died), ACOG claimed, “In the first trimester, the terms miscarriage, spontaneous abortion, and early pregnancy loss are used interchangeably, and there is no consensus on terminology in the literature. However, early pregnancy loss is the term that will be used in this Practice Bulletin”
“Conflating miscarriage management with induced abortion perpetuates stigma, shame, and confusion surrounding pregnancy loss,” the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG) recently wrote. “It is not an uncommon occurrence for patients to call their physician after treatment for a miscarriage, distraught because they saw the word ‘spontaneous abortion’ on their discharge paperwork – causing significant distress because they are opposed to induced abortion.”
In 2022, a petition from 48 pro-abortion groups asked the FDA to approve the abortion pill (mifepristone) for miscarriage care. In addition, the petition sought to convince the FDA to remove mifepristone from an important safety system known as the Risk Evaluation and Mitigation System (REMS).
The letter was signed by the pro-abortion ACOG, the American Humanist Association, American Medical Association (AMA), Expanding Medication Abortion Access (EMAA) Project, NARAL Pro-Choice America, the National Abortion Federation (NAF), Physicians for Reproductive Health (PRH), Planned Parenthood Federation of America (PPFA), and UCSF Bixby Center for Global Reproductive Health, and others likely to benefit financially from the move.
Then, in the fall of 2024, the Wall Street Journal (WSJ) reported that abortion pill manufacturer Danco Laboratories was “preparing scientific data and taking other steps to ask the Food and Drug Administration to approve use of the abortion pill regimen Mifeprex for management of miscarriages, according to people familiar with the matter” (emphasis added).
“It couldn’t be determined when Danco will formally submit its application, though an FDA decision will likely take months,” the WSJ added.
Despite the fact that no approval has been issued yet, ACOG is openly promoting using both drugs in the abortion pill regimen for so-called “early pregnancy loss” or miscarriage care. Planned Parenthood is also promoting use of both drugs on their website.
In its previously mentioned practice bulletin, ACOG described the two-drug regimen for “early pregnancy loss” as “superior,” despite also claiming that “initial studies” were “unclear about the benefit of mifepristone for the management of early pregnancy loss.”
Live Action News reached out to an OBGYN who suggested that there is research to indicate the use of both drugs might have better results. However, it is important to note that as of the writing of this article, there has been no FDA approval of the use of both drugs for miscarriage care.
In addition, we know from abortion industry insiders that getting the abortion pill approved for “other indications” is essentially a scheme to circumvent laws that restrict abortion.

Oddly enough, while ACOG seems to imply that expelling a “nonviable” embryo or fetus in a miscarriage may work best when both drugs in the abortion pill regimen are used, it has also co-signed Big Abortion’s work-around effort to avoid the safety requirements of the REMS for the abortion pill by suggesting that the use of misoprostol only for an induced abortion is acceptable.
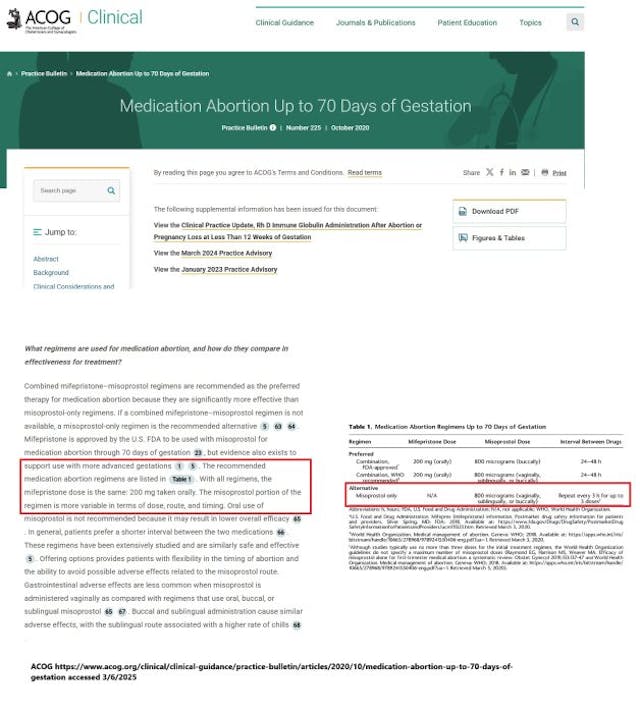
“The majority of [self-managed abortions] SMAs are completed safely with misoprostol, either alone or with mifepristone,” ACOG claimed, adding, “Combined mifepristone–misoprostol regimens are recommended as the preferred therapy for medication abortion because they are significantly more effective than misoprostol-only regimens. If a combined mifepristone–misoprostol regimen is not available, a misoprostol-only regimen is the recommended alternative.”
In their amicus brief filed in the Alliance for Hippocratic Medicine (AHM) v. FDA lawsuit, which went to the Supreme Court, ACOG wrote that “mifepristone followed by misoprostol is used both to induce abortion, and in the treatment of miscarriage or early pregnancy loss (which can be life threatening).” However, the brief also footnoted that “Combined mifepristone–misoprostol regimens are the preferred therapy for medication abortion because they are more effective than misoprostol-only regimens. ACOG Practice Bulletin No. 225, Medication Abortion Up to 70 Days of Gestation, 1, 4 (Oct. 2020) (“ACOG Practice Bulletin No. 225”)” (emphasis added).
What is the approved abortion pill, and how do clinicians obtain it?
Mifepristone (200mg), also sold in the U.S. under the brand name Mifeprex, was approved by the FDA in September of 2000, to be used in a regimen with a misoprostol up to 70 days/10 weeks. (This is an “off-label” use of misoprostol.)
Mifepristone may be used in other medications with separate dosages, but it is the 200mg dosage (2023 label) which was approved for use as the abortion pill. Mifepristone (300mg) is known as the drug Korlym, which is not the abortion pill (label) and is unaffected by any calls to restrict mifepristone. Read more about this here.

The REMS on mifepristone (200mg) limits prescribers to only those who are approved by the “sponsors” of the drugs — Danco or GenBioPro, though the abortion industry wants the REMS removed so it can expand abortion, and sell abortion pills to anyone.
Currently, under the REMS, to become certified, prescribers must have the ability to accurately assess the duration of a pregnancy and diagnose an ectopic pregnancy, and the REMS require providers to sign the manufacturer’s prescribers agreement. Until recently, prescribers also had to stock the drug, since it was not dispensed in pharmacies, but that requirement changed in 2023 when the Biden FDA approved the dispensing of the regimen through retail pharmacies with a prescription.
While the number of prescribers approved by Danco and GenBioPro is unknown, past studies have shown that a majority of OBGYNs were not certified to prescribe the drug, indicating that the majority of OBGYNs would also be unlikely to prescribe mifepristone for miscarriage care.
Abortion pill risks
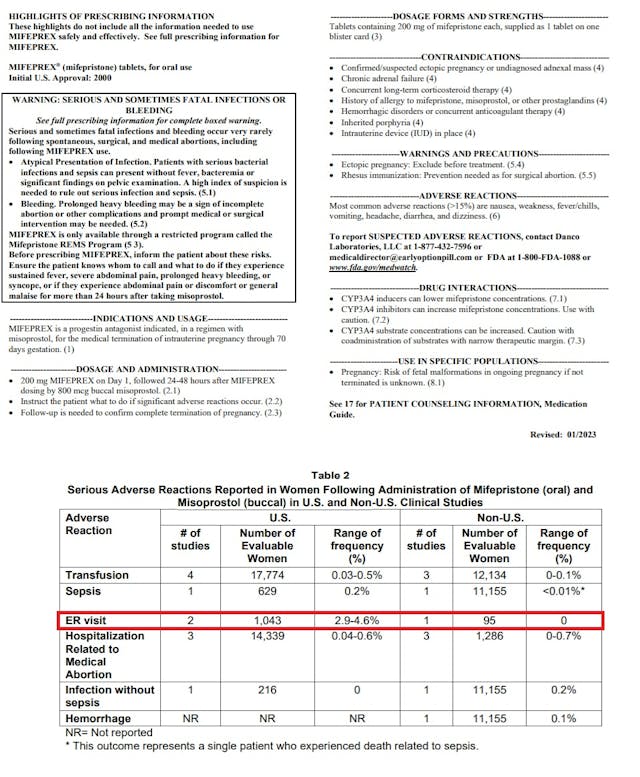
Published percentages for emergency room (ER) visits on mifepristone’s 2023 label state that 2.9 to 4.6% of women who take abortion drugs end up in ER, indicating that these visits could be in the tens of thousands every year. In addition, the FDA’s medication guide acknowledges that as many as seven percent (7%) of women will need surgery after taking mifepristone “to stop bleeding” or to complete the abortion.
The data also is similar to findings from a previously-documented Gynuity Health Projects (GHP) telabortion study, which found that six percent (6%) of participants (70 out of 1,157) faced complications from the abortion pill that resulted in ER or urgent care visits. Gynuity is a pro-abortion group, conducting clinical trials on the abortion pill; it is funded by organizations with deep historic ties to the American eugenics movement.
The “self-managed abortion” scheme was planned and implemented by the abortion industry years before the fall of Roe v. Wade. Even in the early days of the abortion pill’s approval, the industry acknowledged that ERs would be a necessary link in the “self-managed” abortion chain, despite the industry’s repeated claims that the drug is “safe.”
The abortion industry has now shifted responsibility for abortion pill clients from abortion providers to often overcrowded ERs. Today, bad actors inside the industry even tell abortion clients to instead present to ERs when experiencing abortion pill complications, with some even suggesting that clients lie and claim they are experiencing natural miscarriages.
Why does this matter? Because it falsifies the true dangers of the abortion pill for women, instead causing those dangers to be reported as natural pregnancy complications. This then inaccurately skews statistics regarding abortion and pregnancy risks.
To date, the FDA only tracks abortion pill-related deaths that are reported to the manufacturer. Nationally, there is no requirement to report abortion complications to any federal agency. As such, we may never fully know the true risks of this drug regimen.
Who is ACOG?
ACOG claims to be the “premier professional membership organization for obstetrician-gynecologists,” but it is far from unbiased where abortion is concerned. Currently, the group is at the forefront of efforts to expand abortion — in particular, the abortion pill.
ACOG’s shift to abortion advocacy began shortly after its founding, and control of the organization was quickly taken by those involved with Planned Parenthood — the “endemically racist” Population Council and the American Eugenics Society.
In 1960, ACOG fellow Dr. Alan F. Guttmacher, a board member of Planned Parenthood who took the helm as president in 1962, told media that an ACOG committee had begun a fact-finding expedition to liberalize abortion laws. Then, by 1968, its leadership had moved to approve the American Law Institute’s Model Penal Code to decriminalize abortion.
By then, ACOG was led mostly by men connected to eugenics-based organizations.
The organization’s official support for unrestricted abortion on-demand has become more obvious over time. ACOG now refers to abortion (which is the intentional killing of a human preborn child) as “health care,” and a 2023 op-ed written by leaders of ACOG and The Society of Family Planning published in The Washington Post stated that abortion “must be available without restrictions, without limitations and without barriers” (emphasis added).
Read more details here about how ACOG officials and Dr. Guttmacher set out to redefine “conception” and “pregnancy,” and how ACOG officials worked to change the definition of the beginning of life from fertilization to implantation (in the uterus) so that they could redefine contraception for population control purposes.
ACOG has been funded by pro-abortion groups like Ibis Reproductive Health (which has been funded by abortion pill manufacturer Danco Laboratories and Danco investor, the Packard Foundation; read more here about Ibis), the Susan Thompson Buffett Foundation, and the (Bill and Melinda) Gates Foundation.
In return, ACOG has funded the Bixby Center for Global Reproductive Health, where abortionists are trained, even promoting Bixby’s Ryan Residency Programs on its website. The ACOG Foundation, a recipient of taxpayer dollars which claims to fund “programs and activities that further the interests of ACOG members,” has granted Planned Parenthood of California Central Coast $20,000. The Foundation was previously seeking proposals for grants to “advance public education initiatives in a post-Dobbs environment.”
In addition, as Live Action News previously documented, ACOG supports and partners with the abortion industry; it has issued numerous releases, training documents, practice bulletins, and committee opinions supporting abortion. ACOG’s “Guide for Patients Seeking Abortion” even links to websites such as AbortionFinder.org, the National Abortion Federation, and Planned Parenthood — which committed nearly 400,000 abortions last year alone. ACOG has “partnered” with many abortion– and eugenics-focused groups.
Summary
Use of the abortion pill has been unsafe for 6 million preborn children (and counting) who have died because of it, and as documented by the drug’s label, it can potentially lead to complications for the pregnant woman.
Live Action News previously documented how the historical ‘record’ surrounding the approval of the abortion pill has been cloaked in secrecy and hidden from the public for decades with the blessing of the FDA. Were the ‘experts’ associated with pro-abortion organizations or even possibly abortionists? Was the data properly analyzed, or did bias in favor of abortion take precedence over actual safety? We simply do not have these answers, because we have not been given access to the data. And, until the full record on this approval process is revealed, any official expansion of this drug into other areas should be viewed with caution.
The fact remains that while mifepristone (200mg) has yet to be approved by the FDA for miscarriage care, the drug’s safety regulations under the REMS already limits who can prescribe these deadly pills. As such, for decades, miscarriage management has existed effectively without utilizing a drug whose approval thus far has been only to end the life of a living preborn child.
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Angeline Tan
·
Analysis
Cassy Cooke
·
Politics
Bridget Sielicki
·
Human Interest
Andrea Trudden
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·