
A growing number of Americans call themselves ‘pro-choice’ – but what’s really behind it?
Nancy Flanders
·
Investigative·By Catherine Livingston, PhD
Planned Parenthood’s one day second trimester abortion: Better for women, or better for business?
D&E (Dilation and Evacuation) abortions are commonly used for second trimester abortions. These are the abortions that some lawmakers label as “dismemberment” abortions, and they are usually done from 16-22 weeks. They tend to be more complex because of the size of the baby, and therefore, dilating a woman’s cervix for this procedure is also more complex—and it’s also a subject of debate in medicine.
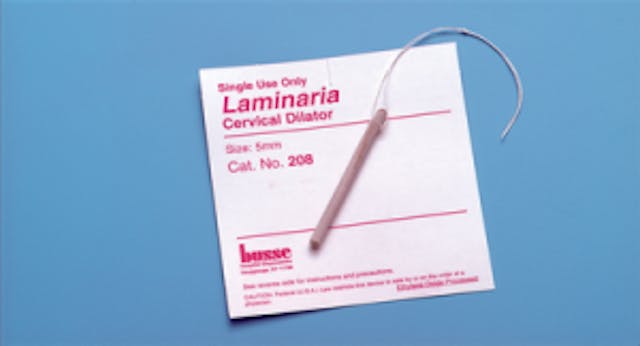
There are two schools of thought on dilating a woman’s cervix prior to a D&E: some use a laminaria (or other osmotic dilator), inserted the day before the abortion; some use buccal misoprostol (which most know as a drug used in medication abortions), given the same day as the abortion.
In the second Planned Parenthood video, Dr. Mary Gatter explains that her Planned Parenthood clinic administers misoprostol for every abortion. And starting at about 38:26, she also notes how the abortionist at her clinic uses a one-day method of cervical dilation for second trimester D&E (Dilation and Evacuation) abortions. Even a 16 week D&E doesn’t get laminaria to dilate for a day prior.
This saves time, allowing the abortion be completed in a single day, even in the second trimester—and that could be an important factor if a clinic only does abortions one day a week. Once a week abortions are often necessitated by having an itinerant doctor who has limited availability. So, in essence, a one-day abortion makes it easier for the clinic.
But is it easier on the women?
This time distinction – one day v. multiple days – has been the subject of numerous medical journal articles over the years, one of which was co-authored by Gatter.
It’s important to note that Gatter’s paper was co-authored with seven other people—and every single one of them worked for Planned Parenthood. In fact, there’s an additional author listed: “Planned Parenthood Federation of America Buccal Misoprostol Waiver Group.” In other words, they have a clear stake in the outcome, and yet, the journal in which they published it, Contraception, is part of the Elsevier journal group, which requires a disclosure of any conflict of interest the parties may have in the research; this is standard in academic journals and is usually published in the article. For example, if a drug company funded research on a drug, even if it was a double-blind study, it still must be disclosed. So it’s important to understand that Planned Parenthood may have had a stake in this research, which is listed as a descriptive study – one that isn’t experimental, but observational.
The Planned Parenthood researchers basically came to the conclusion that the drug was the way to go. They briefly admit that wider research is needed but in reading their study, it’s clear it justifies the use of misoprostol in all cases. In the article, they write (emphasis mine):
Studies have shown that misoprostol can replace laminaria use in some first- and second-trimester abortions.
In summary, our results concerning adequacy and safety of buccal misoprostol in a cohort of 2218 patients are to be interpreted within the context of the limitations of a descriptive study. A descriptive, noncomparative study does not enable determination of whether observed adverse events are caused by preparation method, procedure, provider, patient history or some other reason. It is with caution and in keeping with the limited evidence in the descriptive studies to date that we conclude that women having second-trimester abortions with buccal misoprostol do not appear to be at increased risk of an adverse event or a serious adverse event….
Additional and more rigorous research is needed to identify the safest, most efficient regimen for cervical preparation prior to second-trimester abortion. Buccal misoprostol demonstrates promise to be this cervical preparatory agent.
They are correct in determining this topic needs more research, but drawing a conclusion in a non-experimental study that women “do not appear” to be at extra risk seems a stretch. A brief look at the other literature discussing this indicates the same caution but makes no firm conclusions, though it leans toward osmotic dilators like laminaria.
The December 2007 issue of Contraception also published recommendations from the Society of Family Planning on “Cervical preparation for second-trimester surgical abortion prior to 20 weeks of gestation.” In part, it said, acknowledging limitations of current research:
Routine use of misoprostol as an alternative to osmotic dilation prior to second-trimester D&E is not recommended due to increased risk of inadequate cervical dilation. Buccal or vaginal misoprostol use may be considered by experienced clinicians in lieu of osmotic dilation early in the second trimester (before 16 weeks) in women at low risk for cervical or uterine injury.
Routine use of adjunctive buccal misoprostol in addition to osmotic dilators for preoperative cervical preparation is not recommended in the early second trimester (before 16 weeks) but may be considered at later gestational ages.
Last year, a randomized trial of misoprostol versus laminaria before dilation concluded that while misoprostol can be safely used, there may be additional work, and skilled physicians (not just any practitioners) should be the ones doing it:
Cervical preparation using either laminaria or misoprostol can be safely used before D&E up to at least 19 weeks. Physicians using misoprostol must be skilled at mechanical dilation, since this is commonly required.
In addition, the trial’s results noted there was a significant increase in one side effect for the misoprostol group—diarrhea.
Another randomized trial which compared the use of misoprostol to laminaria for early second trimester surgical abortions concluded:
Early second-trimester abortions take longer and are technically more challenging after cervical ripening with same-day misoprostol than with overnight laminaria, but patients prefer same-day misoprostol… Much larger comparative trials are necessary to evaluate differences in complication rates.
These are a few of the many articles that discuss the unresolved sides of these dilation choices. In short, with the varying research and the more technically challenging aspect and possible risk complications of the one-day procedure, it is irresponsible for Planned Parenthood to have an across-the-board “misoprostol-only” policy for its D&Es. While it seems it might be safe for some, the research with actual randomized trials versus Planned Parenthood’s descriptive study from a decade ago—on which Mary Gatter seems to build a policy in her clinic—is far too mixed to be considered one-size-fits-all.
However, if Planned Parenthood wants to function as an assembly line abortion clinic, with faster results and same-day second trimester abortion services, it has found the way. The question is, is it the best and safest way for the women it claims to care so much about?
The jury is still out on this one, and for now, that could put women in danger.
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Nancy Flanders
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Investigative
Carole Novielli
·
Investigative
Nancy Flanders
·
Investigative
Nancy Flanders
·
Human Interest
Catherine Livingston, PhD
·
International
Catherine Livingston, PhD
·
Newsbreak
Catherine Livingston, PhD
·
Human Interest
Catherine Livingston, PhD
·
Human Interest
Catherine Livingston, PhD
·