A new study on the use of a morning-after pill, described by one pro-abortion advocate as “small,” is the latest in Big Abortion’s obsession to thwart existing U.S. Food and Drug Administration (FDA) safety regulations surrounding the abortion pill regimen.
The emergency contraceptive/”morning-after” pill known as Ella, with the active ingredient of ulipristal acetate, is being promoted in a newly-published study as a possible alternative to the abortion pill (mifepristone). The study claims that utilizing high doses of Ella could be effectively substituted for mifepristone if coupled with misoprostol, an ulcer drug which is also currently utilized as the second drug administered as part of the abortion pill regimen.
Big Abortion has been undaunted in its efforts to expand access to chemical abortion, testing other drugs — even life-saving ones — as potential substitutes for mifepristone, or attempting to extend mifepristone’s use later in gestation, and even suggesting that mifepristone could be used as a contraceptive on its own. The FDA’s recent expansions have been the subject of legal litigation of late, prompting abortion advocates to seek alternative measures to end the lives of preborn babies.
What is the approved abortion pill regimen?
The abortion pill was approved by the FDA in 2000 as mifepristone (brand name Mifeprex) and is administered in a 200-milligram dosage, in a regimen with a second drug, misoprostol. Mifepristone “blocks a hormone called progesterone that is needed for a pregnancy to continue. Mifepristone, when used together with another medicine called misoprostol, is used to end a pregnancy through ten weeks gestation (70 days or less since the first day of the last menstrual period),” the FDA states online.
The FDA eventually placed mifepristone under a safety system (REMS), limiting its use. Despite the concerns, bad actors inside the abortion industry regularly flout these FDA protections. Mifepristone is manufactured under the direction of Danco Laboratories (Mifeprex) and GenBioPro (generic mifepristone).
The latest published data by the FDA revealed that from 2000 through the end of December 2022, “approximately 5.9 million women” used mifepristone in the U.S. for “medical termination of pregnancy.”
What is Ella, and who manufactures it?
Ella’s website calls the drug “an oral emergency contraceptive pill (also known as the morning after pill) containing ulipristal acetate.” This drug was approved by the FDA in 2010 as a “30 mg tablet for the prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure.” Ulipristal acetate “postpones ovulation,” according to the site.
The FDA’s latest 2021 label states that the drug is “a prescription emergency contraceptive [EC] that reduces your chance of becoming pregnant if your birth control fails or you have unprotected sex,” and warns users that the drug is “not indicated for the termination of an existing pregnancy.”

Ella label warns users not to use of pregnant
Ella is the brand name for the drug in the U.S., while EllaOne is the name for the drug in Europe. While the EC is available over-the-counter (OTC) without a prescription in many countries, Ella is easily purchased with a prescription and is advertised readily on websites like Amazon.

BRUSSELS, BELGIUM – APRIL 21: A box of EllaOne (ulipristal acetate 30mg), an emergency hormonal contraceptive treatment manufactured and marketed by HRA Pharma pharmaceutical company, is seen in a pharmacy on April 21, 2023 in Brussels, Belgium… (Photo by Thierry Monasse/Getty Images)
Ella’s 2010 approval was addressed to the Laboratoire HRA Pharma C/o Target Health, Inc. In 2012, changes to the label were addressed to Laboratoire HRA Pharma C/o Watson Laboratories, Inc., which later merged with Actavis Generics. In 2015, Laboratoire HRA Pharma challenged the patent in a lawsuit against Teva Pharmaceutical Industries Ltd.
As of 2021, Watson Pharma (located in New Jersey) and Laboratoire HRA Pharma (located in Paris, France) are listed as manufacturers on the drug’s label. In 2022, “Perrigo Company plc (NYSE: PRGO)” finalized its acquisition of HRA Pharma, “from funds affiliated with private equity firm Astorg and Goldman Sachs Asset Management” acquiring EllaOne.
The Perrigo website currently shows that the company offers Ella and OPill, which is also approved by the FDA as an OTC contraceptive.

Perrigo manufacturers Opill and Ella
Perrigo displays links for an Ella insert, but does not allow the public access to the drug’s insert. EllaOne’s UK packaging and Australian insert claims the EC “does not work if you are already pregnant.” In the U.S., Ella’s website warns prescribers to “[a]dvise patients that they should not take ella® if they know or suspect they are pregnant and that ella is not indicated for termination of an existing pregnancy.”

Ella website warns not to take dug if pregnant
What were the results of the study testing Ella as a potential abortion drug?
Titled “A Proof-of-Concept Study of Ulipristal Acetate for Early Medication Abortion,” the study was published in NEJM Evidence, a New England Journal of Medicine (NEJM) MEDLINE-indexed, peer-reviewed journal. It suggested that “ulipristal acetate followed by misoprostol is an effective and acceptable medication abortion regimen with no reported serious adverse events.”
Without linking to the study, NBC News reported:
In the study, 133 women who were up to nine weeks’ pregnant took a 60 milligram dose of ulipristal acetate, the active ingredient in the prescription contraceptive Ella, followed by misoprostol 24 hours later.
For 97% of them, that drug combo was effective at inducing an abortion, an effectiveness equal to the mifepristone-misoprostol combination. Four women needed a procedure or an additional medication to complete the abortion.
The 60 milligram dose of ulipristal used in the study is twice the dose of Ella, a prescription drug used for emergency contraception.
The company that makes Ella says on its website that it won’t end an existing pregnancy. It can be taken up to five days after unprotected sex to prevent pregnancy.
On Substack, pro-abortion extremist Jessica Valenti referred to the “new small study,” and correctly noted: “But here’s the thing: The study did not find that Ella causes abortion. You cannot take Ella, or its active ingredient ulipristal acetate, to end a pregnancy.”
She added:
What researchers did find was that women could end a pregnancy by taking twice the dose of ulipristal acetate found in Ella, followed by misoprostol—which is a drug that can induce abortion on its own. (As one source put it to me, “Miso and a pretzel can cause an abortion.”)
In other words, it’s not the Ella that’s causing the abortion; it’s the second drug, misoprostol, that’s causing this result. And for quite some time, abortion insiders have been pushing for “misoprostol only” abortions if mifepristone is ever significantly restricted.
According to study authors, the study was based on a “two state clinical trial” to evaluate the “efficacy and acceptability of an ulipristal–misoprostol abortion regimen among participants with pregnancies through 63 days of gestation.”
The study was reportedly “designed by the teams at Gynuity Health Projects and the National Autonomous University of Mexico.”
The study authors claimed (emphasis added):
Like mifepristone, ulipristal acetate binds to the progesterone receptor with high affinity, has good oral availability, and has a wide margin of safety…
Animal studies provide early evidence suggesting that ulipristal acetate has a plausible use for abortion. As an abortifacient, ulipristal reduces progestin levels and has shown efficacy in terminating a pregnancy during the early stages of gestation, comparable to mifepristone.
In humans, ulipristal acetate, when used in doses three times higher than the standard emergency contraception dose and combined with misoprostol, has shown to be a feasible and acceptable regimen for cervical preparation in second-trimester abortions prior to dilation and evacuation.
Gynuity’s website went on to add that participants were “up to 63 days” pregnant when they “swallowed two 30 mg pills” of Ella and then were briefly observed and “discharged with four 200 mcg pills of misoprostol” with instructions to “self-administer the misoprostol 24 hours after the ulipristal, holding two pills in each cheek for 30 minutes before swallowing any remaining bits. Over one week later, they returned to the clinic to determine the status of the abortion and answer a series of questions about their experiences using the study medications and the acceptability of the regimen.”
“Complete abortion occurred with the study regimen in 129 out of 133 (97%) participants, a success rate comparable to a mifepristone-misoprostol regimen. Among those for whom this regimen did not result in pregnancy termination, one had a completion with sharp curettage, two received manual vacuum aspiration, and one underwent a repeat medication abortion with misoprostol alone,” Gynuity wrote.
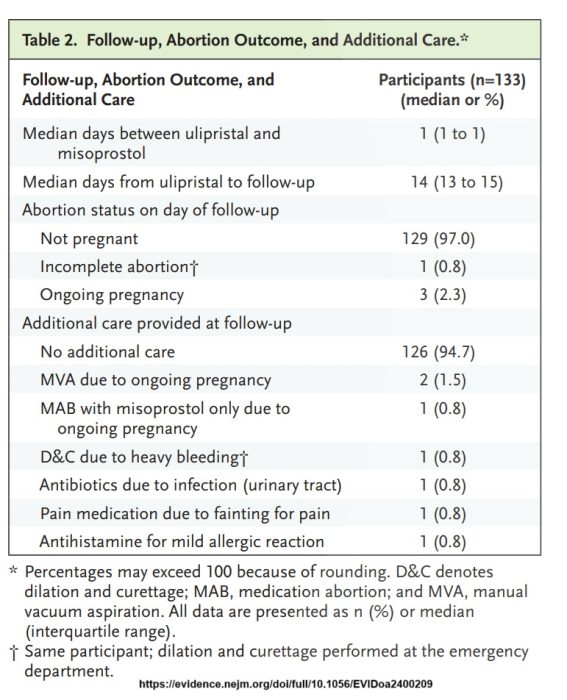
Ella for abortion study outcomes
Gynuity said Ella’s side effects were “rare” and that the misoprostol side effects were mainly “chills, diarrhea, and nausea” which were “transitory, easily managed, and often associated with misoprostol use. No serious adverse events were reported.”
It is important to emphasize that this “small’ study only observed women who were less than 64 days gestation (just over nine weeks) which is less than the FDA-approved 70 days/10 weeks for the abortion pill. Participants were also excluded if they had “confirmed or suspected ectopic pregnancy.” Though not listed in the study, the protocol showed that, unlike today’s no-test abortion pill protocol, study participant’s gestational ages were confirmed by ultrasound beforehand.

Ella for abortion study protocol
“All study participants will have undergone routine screening including ultrasound to confirm intrauterine pregnancy and determine gestational age, and will have received care in accordance with standard practice prior to medical abortion at the study sites…Participants will be required to return to the study site for clinical assessment with a trained team member,” the protocol claimed.
Media cites untrustworthy experts on study
The Atlantic journalist Patrick Adams claimed “Beverly Winikoff, the president and founder of Gynuity Health Projects and a co-author of the study, told me that she knew the stakes when she and her colleagues began their research.”
“In Winikoff’s view, another potential option for medication abortion in the U.S. was too important to ignore,” Adams wrote.
Live Action News previously documented Winikoff’s shocking statement crediting the 9/11 terrorist attacks with saving the abortion pill by drowning out the news of a woman’s abortion pill death.
Another “expert” cited by Adams was the ever-popular abortionist Daniel Grossman: “In an NEJM Evidence editorial accompanying the Gynuity study, Daniel Grossman, a professor of obstetrics, gynecology, and reproductive sciences at UC San Francisco [UCSF], wrote, ‘There is a risk that the findings of this study could be misapplied and used by politicians to try to restrict ulipristal for emergency contraception.'”
Grossman teaches abortion at UCSF and has been a principal investigator in abortion pill clinical trials, even advocating for over-the-counter dispensing of the drug. Grossman has multiple pro-abortion ties and is senior adviser at Ibis Reproductive Health, which — as recently as 2020 — was directly funded by abortion pill manufacturer Danco Laboratories and is currently funded by the Packard Foundation, a large investor in Danco.
Grossman once suggested that medical personnel at emergency departments and ERs should falsify documents to cover up abortion pill complications.
Study designers and funders
The Ella study was designed in part by Gynuity Health Projects (a.k.a. GHP Solutions LLC), a sponsor of United States abortion pill clinical trials and studies (including the mail-order TelAbortion trial). Gynuity is heavily funded by the Packard Foundation, an investor in abortion pill manufacturers Danco Laboratories and GenBioPro.
According to the UK’s registry, the study was funded by The Options for Pregnancy Termination Innovation Initiative (‘OPTions Initiative’). Gynuity wrote online, “This project was supported by the OPTions Initiative and the study was undertaken together with the National Autonomous University of Mexico and Mexico City Health Secretariat.” The OPTions Initiative website claims the group funds “bold, innovative, transformational and ‘outside-the-box’” ideas that “Introduc[e] or increas[e] early access to manual vacuum aspiration (MVA) and/or medical abortion (MA),” including:
- Decentralizing and de-medicalizing abortion care
- Reclassifying existing abortion methods to fit within relevant regulatory frameworks
- Reframing and normalizing abortion to combat stigma
- Increasing the number of trained providers and available safe services
Study authors
Authors of the Ella study included:
- Beverly Winikoff, M.D.: Founder of Gynuity who previously worked for the Population Council, which brought the abortion pill (known then as RU486) into the U.S. Winikoff has served on the board of the National Abortion Federation (NAF), which was at one time directly funded by Danco, as well as the Buffett and Packard Foundations (early investors in Danco).
- Manuel Bousiéguez: Staff at Gynuity; main contact for the Ella study.
- Jorge Salmerón, M.D., Sonia Hernández-Salazar and Karina Robles-Rivera, M.D.: Associated with the National Autonomous University of Mexico, Mexico City.
- Angélica Martínez-Huitrón, M.D., Lucía Aguirre-Antonio, M.D, and María Laura García-Martínez, M.D.: Associated with the Mexico City Health Secretariat, Mexico City; García-Martínez was the principal investigator of the study.
- Ilana G. Dzuba: Former senior advisor at Gynuity; LinkedIn shows she is currently employed by Ariadne Labs. In a 2015 commentary published in Contraception, Dzuba praised the off-label indications for the abortion pill mifepristone.
In addition, Daniel Davis, M.D., Senior Consultant at FDA, and abortionist Matthew Reeves (founder of DuPont Clinic — a late-term abortion facility — and a former medical director of the NAF), served as advisors.
Pro-life Reaction
“Mifepristone and Ella are both selective progesterone receptor modulators, meaning they block progesterone in the woman’s body, which would prevent an implanted embryo from receiving the nutrients needed to survive,” the Association of Pro-life Obstetricians and Gynecologists (AAPLOG) wrote on X.
🚨BREAKING NEWS: After years of denying this emergency contraceptive’s potential to end an embryo’s life, abortion advocates are now using it as a substitute for the abortion drug mifepristone. 🧵
1/4
— AAPLOG (@aaplog) January 23, 2025
Dr. Donna Harrison, AAPLOG’s founder and director of research, has been looking into the science of Ella for a number of years. In 2010, she warned ABC News that Ella was “a thinly veiled attempt to get an abortion drug over-the-counter.”
“To label this as emergency contraception when it’s clearly an abortive action is dishonest,” said Harrison.
Live Action News previously detailed the pro-abortion American College of Obstetricians and Gynecologists’ move to redefine the beginning of human life from fertilization to implantation for the purpose of deceptively marketing abortifacients as birth control.
In response to the recent study, Dr. Harrison told the New York Times, “After years of denying ulipristal acetate’s potential to end the life of an embryo, abortion advocates are now starting to use it as a substitute for the abortion drug mifepristone. The reason for this is simple. Ulipristal and mifepristone function in the same way.”
“Women deserve to be fully informed about how this drug works, as well as its risks,” Harrison told The Atlantic.
Dr. Ingrid Skop, an obstetrician gynecologist associated with the pro-life Charlotte Lozier Institute (CLI), told Live Action News, “Ella is approved as a contraceptive, not an abortion drug. This study that shows an intention to use Ella as an abortion drug gives cause to reexamine how Ella is regulated and funded. Misusing Ella would not only end the lives of unborn children but increase unsupervised abortions that can pose major medical risks to women.”
Tell President Trump, RFK, Jr., Elon, and Vivek:
Stop killing America’s future. Defund Planned Parenthood NOW!







