Pro-life groups are questioning the timing of Sage Publishing’s decision to retract three studies where the authors had “affiliations with pro-life advocacy organizations” including the Charlotte Lozier Institute (CLI). Two of those studies are cited in an abortion pill lawsuit set to be heard before the United States Supreme Court in March.
The three now-retracted studies were led by researcher James Studnicki (who reportedly trained at Johns Hopkins University) and published in Sage’s journal “Health Services Research and Managerial Epidemiology.” Two were funded by the Charlotte Lozier Institute (CLI), the research arm of the pro-life organization Susan B. Anthony Pro-Life America. In addition, several of the authors were also associated with CLI.
Today, @Sage_Publishing retracted 3 of our studies, citing “methodology concerns” & “undisclosed conflicts of interest.”
All 3 use Medicaid records-linkage data, the highest quality abortion-related data available in the US@leif_lemahieu @realDailyWire:https://t.co/sNVhdaLK9J
— Charlotte Lozier Institute (@LozierInstitute) February 5, 2024
Sage, which describes itself as “the world’s leading independent academic publisher… committed to global dissemination of research…” has denied that the timing of the case played a role in the retractions.
CLI disagrees — and in a long Twitter/X thread, called the move an “assault on science” — even publishing a new website to respond to the critics.
🧵 On February 5th, @Sage_Publishing announced it would retract three of our studies.
All three use Medicaid records-linkage data, the highest quality abortion-related data available in the U.S.
This should be of great concern to the entire research community.
1/15 pic.twitter.com/b8Mi6IqPhD— Charlotte Lozier Institute (@LozierInstitute) February 7, 2024
Studies Cited in Abortion Pill Lawsuit to be Heard before SCOTUS
Two of the three retracted studies are cited by plaintiffs in the Alliance for Hippocratic Medicine [AHM] v. Food and Drug Administration (FDA) lawsuit which calls into question the 2000 approval of the abortion pill mifepristone by the FDA. In addition, both studies were later cited in the decision by District Court Judge Matthew J. Kacsmaryk to suspend FDA approval of the drug.
The case is set to be heard before the Supreme Court in March and will focus on the legitimacy of the 2016 and 2021 changes to the FDA’s REMS safety system regarding mifepristone.
“On November 16, the researchers sent a letter to Sage requesting more time to respond to the retraction notice, saying that the retractions were ‘procedurally improper’ and suggesting that the real reason for the retractions was to undermine their research ahead of the Supreme Court’s consideration of mifepristone,” noted the Daily Wire (DW).
The study, “A Longitudinal Cohort Study of Emergency Room Utilization Following Mifepristone Chemical and Surgical Abortions, 1999–2015” is cited on page 19 of the AHM complaint. It reads, “Chemical abortions are over fifty percent (50%) more likely than surgical abortions to result in an emergency department visit within thirty days, affecting one in twenty females. The number of chemical abortion-related emergency room visits increased by over five hundred percent (500%) between 2002 and 2015.”
In addition, “A Post Hoc Exploratory Analysis: Induced Abortion Complications Mistaken for Miscarriage in the Emergency Room are a Risk Factor for Hospitalization” is cited on pages 20-21 (line 70). It reads: “If a chemical abortion is miscoded as a miscarriage in the emergency room (which occurred sixty percent (60%) of the time in one study), the treating doctor’s lack of knowledge results in the woman or girl being at significantly greater risk of needing multiple hospitalizations and follow-up surgery.”
Claim: Data is misleading
While the Sage retraction criticizes three studies tied to CLI, the pro-life authors have disputed the claims “[i]n a point-by-point response to Sage’s critiques of the paper sent to the publisher in November…” Retraction Watch reported.
The authors have made clear to Sage that the retractions are “unwarranted”:
- No finding in any of the three studies has been substantively challenged, let alone proven invalid.
- Sage has shown no evidence of any major errors, miscalculations, or falsehoods.
- There is no breach of the guidelines of the Committee on Publication Ethics, which helps set standards for academic publications.
- These retractions are clearly unwarranted.
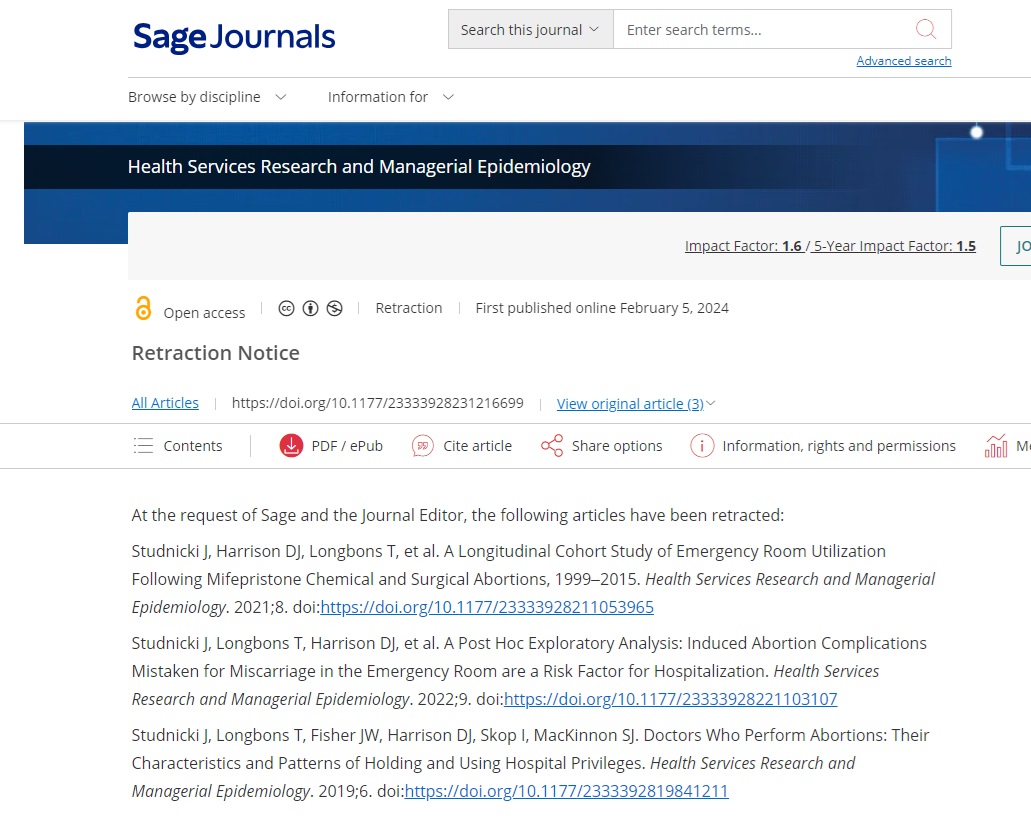
SAGE Publication retraction notice lists three Charlotte Lozier studies on abortion
The 2021 study entitled “A Longitudinal Cohort Study of Emergency Room Utilization Following Mifepristone Chemical and Surgical Abortions, 1999–2015” looked at Medicaid data to measure emergency room visits and complications after abortions.
According to the CLI website, the study concluded that “the rate of abortion pill-related emergency room visits has increased more than 500% over the past decade and a half, and that abortion pills put mothers at significantly greater risk for complications.”
Critics of the study claimed it artificially inflated the number of adverse events because the authors counted multiple visits by the same patient as multiple visits.
But in their rebuttal to Sage, the authors wrote: “If a woman has three emergency room visits on different days within 30 days of an induced abortion, and all are reported, that is not ‘artificially inflating the number of adverse events.’ Rather, it is an accurate count of the number of adverse events.”
“Studnicki said that none of the issues raised by Sage invalidate the research. He said the concerns are just a matter of researchers deciding to measure things in different ways, and not a matter of faulty data,” DW reported.
“So, the strength of this design means that this study gave us a really powerful look at what has been happening with chemical abortion… in the United States over the past couple of decades,” study author Tessa Longbons stated.
Another criticism of that study was directed at the use of emergency room data — and again, the pro-life authors addressed the issue, writing to Sage that “The rationale for our study of ER use following abortion is extensively discussed in the introduction to our paper, and the reviewers did not provide any rebuttal to this explanation.”
The authors also noted that “adverse events following a mifepristone abortion are more likely to be experienced at home in the absence of a physician, increasing the likelihood of an ER visit.”
The study findings “demonstrate[] that some women go to the emergency room after they’ve had a chemical abortion… with bleeding and they do not communicate to the emergency room physician that they had the abortion,” Studnicki said in a response video.
Live Action News previously documented how abortion providers often tell women that if they need to report to the ER when suffering an abortion pill complication, they should lie to ER staff and instead claim they are experiencing a natural miscarriage.
This clear deception not only potentially puts the woman at risk for proper care but essentially hides abortion pill complications.
The issue of abortion pill clients reporting to emergency rooms for care was discussed in the lawsuit as a reason AHM plaintiffs had standing in the case, which leads some to — again — question the timing of the Sage retractions.
District Court Judge Kacsmaryk was clear in his 67-page opinion that not only did the plaintiffs have standing in this case (pp. 7-11, 11-13), but plaintiffs’ alleged injuries are concrete and redressable (pp. 13-17) and within the statutory zone of interests (pp. 16-18).
Kacsmaryk wrote (p. 7) that “Here, the associations’ members have standing because they allege adverse events from chemical abortion drugs can overwhelm the medical system and place ‘enormous pressure and stress’ on doctors during emergencies and complications.”
“These emergencies ‘consume crucial limited resources, including blood for transfusions, physician time and attention, space in hospital and medical centers, and other equipment and medicines,’” the District Court judge added, linking those decisions directly to the aforementioned study.

AHM lawsuit on Standing in District Court Decision
“The study has been widely cited and has provided impetus for other studies seeking better information on the impacts of abortion on women. Yet a single as-yet-unpublished complaint prompted Sage to apply an Expression of Concern (EOC) to the article,” CLI wrote on X, adding, “The Expression of Concern did not refer to any finding of the study. Instead it took issue with the presentation of data – which had been previously accepted by Sage reviewers and editors – and ‘author conflicts of interest.'”
“Sage had since heard Dr. Studnicki’s explanation of the data and admitted that the presentation is not unusual. But, instead of responding to his answers, Sage announced that it was retracting 3 studies. Then, they revoked Dr. Studnicki’s membership on the Health Services Research and Managerial Epidemiology’s (HSRME) editorial board (HSRME is a journal in the Sage network),” CLI also noted.
Claim: Conflicts of Interest not Disclosed
Sage’s retraction notice claims that a “reader contacted the journal with concerns” about the studies because “the authors’ affiliations with pro-life advocacy organizations, including Charlotte Lozier Institute, present conflicts of interest that the authors should have disclosed as such in the article.”
Sage confirmed that “all but one of the article’s authors had an affiliation with one or more of Charlotte Lozier Institute, Elliot Institute, and American Association of Pro-Life Obstetricians and Gynecologists, all pro-life advocacy organizations, despite having declared they had no conflicts of interest when they submitted the article for publication or in the article itself.”
But in their response to Sage, the pro-life authors claimed they “completely and accurately disclosed all affiliations and financial support as part of the online submission process.” And they claimed that Sage’s alleged conflicts of interest concerns “are not only inapplicable to our authorship, but have not been applied to any other set of authors publishing on similar topics in Sage’s family of journals.”

Sage retracted abortion pill study partly due to pro-life authors
As seen in the image above, the authors’ past and present affiliations were noted in the author biographies included in the study.
“The ER study includes 10 mentions of CLI and the authors’ professional status there. However, Sage now claims that CLI authors and scholars involved in the studies had to report their employment at the pro-life organization as a ‘conflict of interest,’” CLI wrote on X.
Sage Double Standard?
“Sage journals have published dozens of articles on abortion access and safety by researchers affiliated with organizations with very public and open positions on abortion,” the response to Sage read.
The pro-life authors then cited “A study on abortion safety in which the sole author [Susheela Singh] is affiliated with Guttmacher Institute” where “No conflicts were disclosed.” The Guttmacher Institute (named after Dr. Alan F. Guttmacher, a former VP of the eugenics society and one-time president of Planned Parenthood) is also a former “special affiliate” of abortion corporation Planned Parenthood. Guttmacher openly advocates for abortion.
There are no disclosures in this study by the pro-abortion Guttmacher Institute: https://t.co/0Gjb1kEZQ7
Or this study led by an abortionist (with all authors affiliated with the Bixby Center for Global Reproductive Health): https://t.co/YWWUWhG7ED
12/15— Charlotte Lozier Institute (@LozierInstitute) February 7, 2024
Another Sage study on the abortion pill was authored by Kate Grindlay, who worked at Ibis Reproductive Health at the time. In 2016, when this study was published, Ibis’ website acknowledged the organizational focus was “increasing access to safe abortion.” It also listed abortion pill manufacturer Danco as a “funder and strategic partner.” In fact, as late as 2020 (when the second author, Daniel Grossman served as Ibis’ senior adviser) Ibis had still been receiving funds from Danco Laboratories.
Author Grossman listed his associates as Advancing New Standards in Reproductive Health (ANSIRH) and the Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Francisco, CA, claiming no conflicts as well.
ANSIRH’s website claimed to be a “collaborative research group at the University of California, San Francisco (UCSF)’s Bixby Center for Global Reproductive Health,” which trains abortion providers through its Ryan Residency Training Program. ANSIRH, which was founded in 2002 by abortionist Felicia Stewart, also publishes workbooks on abortion training. Bixby and ANSIRH have both been funded by the Packard Foundation, a large investor of Danco. And the Buffett Foundation, a known collaborator in abortion pill clinical trials, has also invested in the University of California system.
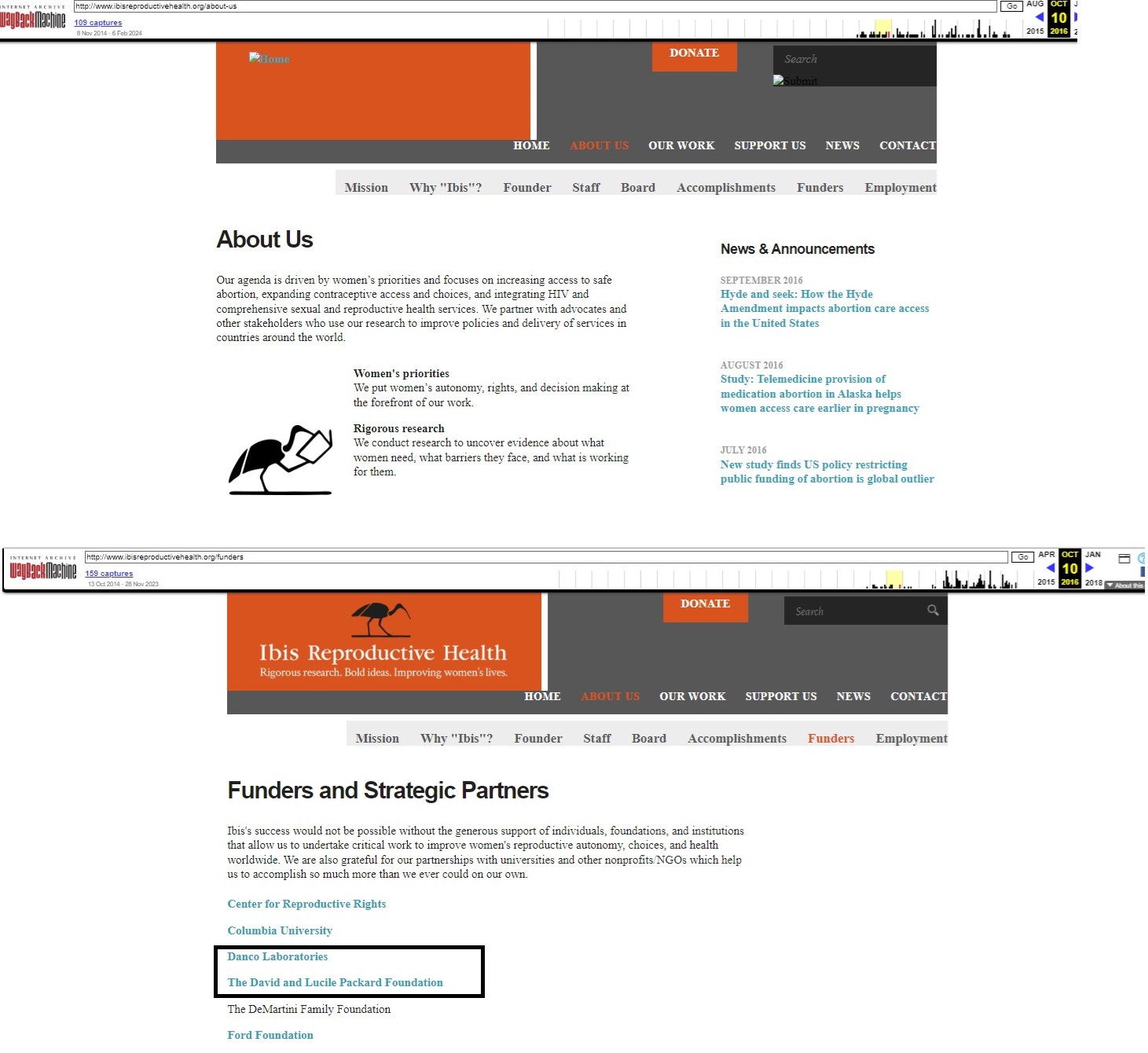
Ibis Reproductive Health promotes abortion funded by Danco and Packard 2016 WBM
A study on so-called self-managed abortion published by Sage lists Abigail Aiken as one of the authors. Aiken is a member of the Society of Family Planning (SFP), which funded the research. SFP was founded in 2005 thanks to a generous contribution from the Packard Foundation, heavily funded by the Buffett Foundation, both investors in abortion pill manufacturer Danco.
Sage’s attention to conflicts lacks in who they “partner” with as well, which includes the National Academies of Sciences Engineering and Medicine (NASEM), whose 2018 report on abortion, “The Safety and Quality of Abortion Care in the United States” is heavily cited. That report funded by NASEM was also funded by multiple pro-abortion philanthropists, including the David and Lucile Packard Foundation and the Susan Thompson Buffett Foundation — both early investors in the abortion pill. It was also funded by the Grove Foundation, the JPB Foundation, the Tara Health Foundation, and the William and Flora Hewlett Foundation, the last of which not only funds an abortion training program but also funds the abortion chain Carafem.
When asked about the double standard, Sage told DW that the assertion was “baseless and needlessly provocative.”

CLI Assault on Science website points to a double standard from Sage publications
Additional examples are mentioned on CLI’s website here.
Claim: Peer Reviewer Affiliated With CLI
“As a result of Sage’s inquiry into the authors’ conflicts of interest, Sage became aware that a peer reviewer who evaluated the article for initial publication was also affiliated with Charlotte Lozier Institute at the time of the review. In accordance with the Committee of Publication Ethics (COPE) standards, Sage and the Journal Editor determined the peer review for initial publication was unreliable. This reviewer also peer reviewed two other articles by the same lead author, published in the journal in 2022 and 2019, which also are the subject of this notice,” Sage alleged as part of their retraction.
In their response to Sage, the authors pointed out that “Sage had and has entire and exclusive control of the review process including the selection of the reviewers, who are unknown, as is appropriate, to us. The retraction notice declares that one of those reviewers is identified as an associate scholar of the Charlotte Lozier Institute (CLI). These scholars are generally not employees of CLI. They are simply scholars who generally agree with or support CLI education and research efforts. We did not select the reviewer at issue, and the reviewer’s identity is still unknown to us. In fact, this reviewer may not have been a CLI associate scholar at the time of his/her review. Likewise, because Sage practices double-blind peer review, the reviewer was equally unaware of our identities when evaluating our work,” the author’s response stated (emphases added).
Hypocrisy of Media and Scientific Community
While multiple media outlets reported the retracted studies by pro-life scholars, the media seems unwilling to entertain any possibility that abortion pill investor dollars potentially taint the claim that the abortion pill is safe.
And they have been silent on previously detailed pro-abortion studies that have hidden funding sources.
In addition, the media seems to willfully turn a blind eye to the multiple conflicts of interest surrounding abortion pill clinical trial sponsors, and they fail to take a critical look at pro-abortion study authors even though some are on the payroll of the manufacturers of the drug.
“All of the major health associations are pro-abortion, most of the journals are pro-abortion, all the academic departments in the universities are pro-abortion,” Studnicki told DW.
“They were making the case that abortion is ‘safe.’ For them it was fixed science,” he said in the previously linked video. “And some of our work, especially some of the work on emergency room visits, represents a different point of view than the narrative they thought was secure.”
Studnicki called Sage’s retractions “a blatant attempt to discredit excellent research which is incongruent with a preferred abortion narrative,” Retraction Watch wrote.
He also described the retractions as “completely unjustified” and told DW that the pro-life researchers were targeted “because of the visibility of our work, because of the fact that our work was having such an influence on the discussion about abortion that was occurring in the states and in the courts at the highest levels.”
On X, CLI claimed that “none of Sage’s methodological concerns invalidate *any* of the findings of the three studies. Rather than engaging on the science, Sage has decided to act as judge, jury, and executioner, ignoring CLI’s affirmative, good-faith defense of its research.”
CLI summed up, “We stand by our work, and we stand behind Dr. Studnicki and his eminent career. If this precedent of rejecting open inquiries in the scientific field continues, then the honest pursuit of science has truly become an afterthought.”







