The historical ‘record’ surrounding the approval of the abortion pill (mifepristone/Mifeprex) has been cloaked in secrecy and hidden from the public for decades with the blessing of the U.S. Food and Drug Administration (FDA) — prohibiting plaintiffs involved in abortion pill lawsuits as well as the American taxpayers from learning which purported experts originally reviewed and later approved the abortion pill.
Even today, 25 years later, the public still has little information about the abortion pill’s approval process in the United States. Were the ‘experts’ associated with pro-abortion organizations or even possibly abortionists? Was the data properly analyzed, or did bias in favor of abortion take precedence over actual safety? We simply do not have these answers, because we have not been given access to the data.
Until the full record on this approval process is revealed, the American public should continue to question the purported safety of self-managed abortion for women; we already know it has been unsafe for six million preborn children and counting, who have died because of it.
KEY TAKEAWAYS:
- The FDA’s original approval process for the abortion pill that began in the mid-1990s has involved an unprecedented amount of secrecy regarding its manufacturer, the manufacturer’s location, its financiers, and its reviewers — who eventually approved its use.
- The mainstream media was suspicious of the secrecy surrounding the approval process in the 1990s and 2000s; the media today regurgitates the abortion industry’s talking points with no scrutiny.
- Experts at the time noted that it was unheard of for the FDA to refuse to disclose a drug’s reviewers or its manufacturer.
- Evidence points to the idea that the abortion pill was approved as a tool of eugenic population control; this evidence includes a letter from a Roe v. Wade attorney to newly-elected President Bill Clinton.
- Documentation surrounding the abortion pill’s original approval process is so difficult to obtain that not even a Federal Appeals Court succeeded — with attorneys unable to give a date as to when (or if) that documentation would be provided.
How the abortion pill came to the U.S.
Mifeprex (mifepristone 200mg) was approved by the FDA in September 2000 “for medical termination of pregnancy.” Generic mifepristone was approved in April 2019. But the drug’s approval process began six years earlier, in 1994, when the Bill Clinton Administration pressured French pharmaceutical company and patent owner Roussel-Uclaf to assign the U.S. rights of marketing and distribution of the abortion pill (under the name RU-486) to the eugenics-founded Population Council.
The right to distribute the drugs was later granted to Danco Laboratories, a sub-licensee of the Population Council. Then, by 1996, the Population Council (funded in part with investments from the Susan Thompson Buffett (Warren Buffett) and David and Lucile Packard Foundations) submitted its application for the drug to the FDA — and a series of clinical trials began.
The FDA initially approved the drug for use in chemical abortions up to seven (7) weeks of pregnancy in a regimen along with a second drug called misoprostol. As the years went on, use of the drug was expanded multiple times — enabling the drug to be prescribed up to 10 weeks/70 days gestation, removing the in-person requirement for dispensing the pill, and more recently, allowing for mail-order and pharmacy dispensing of the drug.
Today, Bad Actors inside the abortion industry openly flout these FDA regulations.
Secrecy surrounds abortion pill’s politicized approval process
Secrecy surrounding the abortion pill’s approval process began in the 1990s during clinical trials, and extended into the 2000s. The FDA essentially politicized the process during the early days of approval, in several ways:
- The abortion pill’s manufacturer was kept secret.
- The location of Danco’s manufacturing plant remains elusive even today.
- Many of the abortion pill’s financiers remain secret, albeit some are funding abortion pill studies, location sites, Journals and study authors.
- The FDA’s approval process was kept secret, with the agency choosing not to publish the names of experts who reviewed the drug.
Author Julie A. Hogan, who wrote a historical review of the process, said, “In return for working with the drug, companies wanted confidentiality. The FDA and the Population Council, in an unusual move, agreed to provide the desired secrecy.”
When the Family Research Council (FRC) analyzed the abortion pill’s original approval process, it noted how FDA Commissioner David Kessler, M.D., who chaired the 1996 Advisory Committee, was later given a lifetime achievement award by NARAL Pro-Choice America. Is the public to believe that there is nothing important to see here?
FRC’s report, “Politicized Science: The Manipulated Approval of RU-486 and Its Dangers to Women’s Health,” additionally pointed out that Kessler was also among those within the Clinton administration who pressured Roussel Uclaf to grant marketing and distribution rights of RU-486 to the Population Council.
The report found that eight of the 11 members who approved the drug “were either affiliated with an abortion organization or ha[d] made pro-choice statements in the past.”
Media scrutinizes secrecy of the process
During initial trials, the media was stunned at the amount of secrecy surrounding the abortion pill; today, the media sticks to the talking points of abortion insiders, even hiding their ties to the abortion pill manufacturer and investors.
In 2000, the New York Daily News wrote:
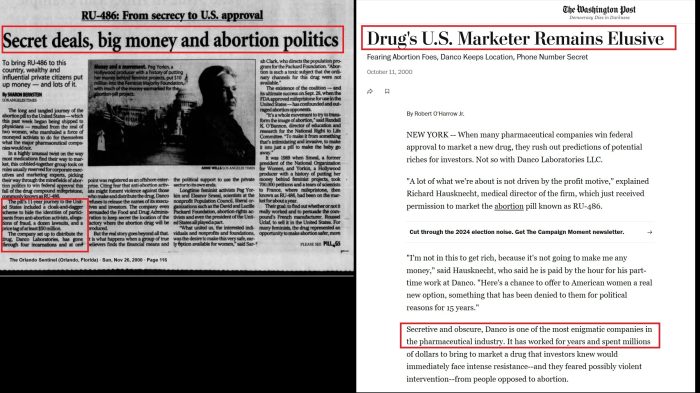
“The pill’s 11-year journey to the United States included a cloak-and-dagger scheme to hide the identities of participants…allegations of fraud, a dozen lawsuits, and a price tag of at least $50 million….
Citing fear… Danco refuses to release the names of its executives and investors. The company even persuaded the Food and Drug Administration to keep secret the location of the factory where the abortion drug will be produced….
In a strange twist, the FDA acceded to Danco’s request that the name of its manufacturer be kept secret — and even shielded the names of the FDA researchers who had overseen the pill’s approval.”
The allegations were repeated by the Orlando Sentinel in the article, “Secret Deals, Big Money and Abortion Politics,” as well as in the Los Angeles Times, among others.
Abortion pill secrecy
No Names: Secrecy surrounds identities of ‘experts’ who approved abortion pill
In 1996, the New York Times wrote about the security and secrecy of the eight-member committee of advisers to the FDA who recommended “the agency approve for marketing the abortion-inducing drug RU-486, or mifepristone,” describing them as “physicians specializing in obstetrics and gynecology.”
Its chairman was Dr. Ezra C. Davidson Jr., a professor of obstetrics and gynecology at the Charles R. Drew University of Medicine and Science in Los Angeles,” the NYT wrote. Davidson had served as president of the extremely pro-abortion American College of Obstetricians and Gynecologists (ACOG) from 1990-1991.
“The panel nonetheless recommended approval, in a series of three votes: By 6 to 0, with 2 abstentions, it decided that the drug’s benefits outweighed its risks; by 7 to 0, with 1 abstention, it decided that the drug was safe, and by 6 to 2 it decided that data from a French study were sufficient to support American use of the drug.”
In 2000, the Washington Post wrote that “FDA Commissioner Jane E. Henney said the agency broke with precedent by not publishing the names of the experts who reviewed RU-486 for the agency (emphasis added). In another first, it did not publish the name or location of the company that will manufacture the drug.”
That manufacturer was also kept secret — something that University of Florida drug law expert Lars Noah told the New York Times in 1996 was unprecedented. “I’ve never heard of a situation where, with the blessing of the F.D.A., you could keep the manufacturer of a drug secret,” he said.

FDA secrecy on abortion pill and record still hidden
Two decades later (May 2020) the ‘record’ about the abortion pill had still not been released, when Columbia University journalists Lauren Mascarenhas and Abigail Brone interviewed early abortion pill advocates. “Because of the controversy surrounding mifepristone, the Population Council [which brought the pill into the United States and set up Danco], kept this distributor a secret throughout the process,” they wrote.
According to Mascarenhas, “By the summer of 1996, the time had come for an FDA advisory committee to meet and decide whether to recommend mifepristone for marketing in the U.S.” She noted that “[a]dvisory committees don’t make the final decision,” adding that the Population Council “knew that securing a recommendation all but guaranteed eventual FDA approval.”
Interestingly, while most of the public has no access to the record, these two Columbia University journalists were able to locate one of the FDA’s senior medical reviewers who reportedly “chose to remain anonymous” but admitted, “It’s definitely not standard. It’s not routine; you can look up almost every other drug that I was the primary medical officer for and my name would appear right there on the review.”
The journalists noted that they did look up this anonymous reviewer’s name and found it “listed on at least eight other FDA drug reviews. But no staff names are listed on the review of mifepristone.”
Experts who reviewed the drug for the FDA remain anonymous; we have no way of knowing whether bias or conflicts of interest played a role in their approval of the intentionally deadly drug.
The suspected ‘why’ behind the abortion pill
A 1991 book published by three pro-abortion researchers suggested that the abortion pill was developed to restrict population growth in minority countries — an example of eugenics in action.
“The specific intention of such research was to restrict population growth in countries that were judged to be ‘underdeveloped.’ If successful, the method(s) could be extended to groups in the United States, Black, Hispanic and Native American Women (Department of Health, Education and Welfare, NIH, USA, 1969),” the authors of “RU 486 Misconceptions Myths and Morals” wrote.
Then in 1992, Ron Weddington, co-counsel in the Roe v. Wade case and the ex-husband of pro-Roe attorney Sarah Weddington, wrote to the newly-elected Clinton administration. In that letter, uncovered by Judicial Watch, he expressed concern about the growth of certain population groups, suggesting that President Bill Clinton should “eliminate” certain segments of society via vasectomies, tubal ligations, abortions, and the RU-486 abortion pill (emphasis added):
Sarah and I have been discussing the notion of our setting up [a non] profit corporation to license and distribute RU486. It’s possible that such an endeavor could be the vehicle for a number of birth control efforts. 26 million food stamp recipients is more than the economy can stand.

Ron Weddington letter about RU486 abortion pill to President Clinton cover page (Image: Judicial Watch)
In 1986, abortionist Warren Hern, who was then the director of the late-term Boulder Abortion Clinic in Colorado, published skepticism about RU-486. Ten years later, in 1996, Hern suggested to the New York Times that “some within the abortion rights movement had exaggerated the drug regimen’s ease of use while glossing over its side effects, and that it had become politically incorrect to even question their view.”
Hern reportedly claimed, “A lot of people in the pro-choice movement see this drug as a panacea. They have a tremendous amount of ideological fervor.”
Then, in 2000, the New York Times cited Hern as saying. ”There is no question that mifepristone is highly effective and there are good results most of the time.” Citing side effects such as incomplete abortions, he added, “people have a way of denying these problems with the drug and saying it’s a magic bullet. It’s not.”
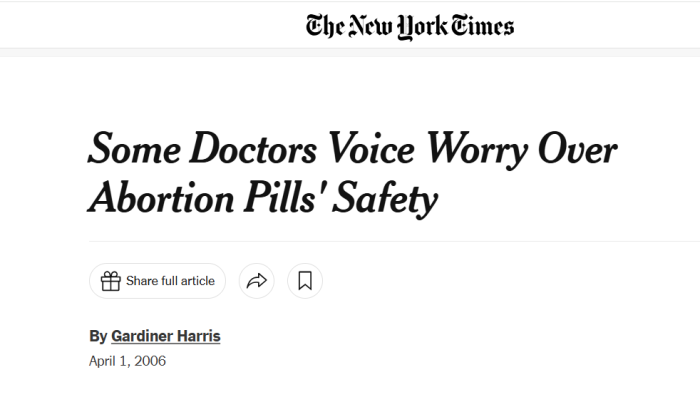
In 2006, following the deaths of multiple women who took the drug, the New York Times reported that Hern said these deaths “demonstrated that abortions by RU-486, or Mifeprex, were far riskier than surgical ones.” Hern said, “I think surgery should be the procedure of choice,” adding that pills “are a lousy way to perform an abortion.”
In that same article, abortionist Damon Stutes of Reno, Nevada, stated his refusal to provide the abortion pill because “The complications associated with RU-486 far exceed the complications of surgical abortion.”
Abortionists skeptical of abortion pill safety New York Times
As late as last fall, Warren Hern was still questioning the care that women receive when they are sold DIY abortion pills. He asked who is doing “follow-up exam[s]” and “tak[ing] care of… a complication.” The answer, often, is that urgent care centers or emergency rooms are left to treat women’s complications.
Even courts remain in the dark
During oral arguments in the case Alliance for Hippocratic Medicine (AHM) v. Food and Drug Administration (FDA) in 2023, a tense exchange took place between the FDA’s legal representative and a judge on the United States Court of Appeals for the Fifth Circuit regarding access to relevant documentation about the FDA’s original approval of the abortion pill.
The missing documents likely include correspondence, expert reviews, studies, trials, or data surrounding this often secretive abortion pill approval process.
Judge Jennifer Walker Elrod, one of the three judges hearing the case, confronted FDA legal counsel Deputy Assistant Attorney General Sarah Harrington: “I just wanted to ask quickly about the record. We don’t seem to have the administrative record in this case.” Harrington said she had even “checked with the clerk’s office” that morning.
“We absolutely do not have the administrative record,” the FDA’s attorney confirmed.
“Why don’t we?” the judge asked, insisting that the court “absolutely need[s] it.”
Harrington sounded rattled as she admitted that the FDA had not yet assembled the documents, claiming it was due in part to the fact that there were “hundreds of thousands of pages.”
“Where is it and how do we get it?” Judge Elrod asked.
“The District Court acted before we were able to produce,” Harrington claimed.
“How long will it take you to get it to us?” Judge Elrod asked.
Harrington was unable to give the judge a precise date, which seemed to frustrate the judge.

Judge Elrod scolds FDA legal Counsel Sarah Harrington in AHM v FDA abortion pill lawsuit at 5th Circuit
Following that exchange, the United States Supreme Court ruled that the plaintiff doctors in the AHM lawsuit did not have standing to sue. Now, an October 2024 lawsuit, State of Missouri; State of Kansas; State of Idaho v. FDA, is making its way through the courts with regard to the abortion pill.
After nearly 25 years, we do not have the record nor do we know which ‘experts’ approved the abortion pill. The American public should demand that Sara Brenner, M.D., M.P.H., the Trump Administration’s Acting Commissioner of the FDA (appointed until the Senate confirms Trump’s nomination for Commissioner, Marty Makary, M.D.), clear the path to releasing the full record on the abortion pill so all experts and documents can be examined.