Judge overturns state’s mandate to check for dangerous ectopic pregnancy before giving abortion pill
Bridget Sielicki
·
Newsbreak·By Calvin Freiburger
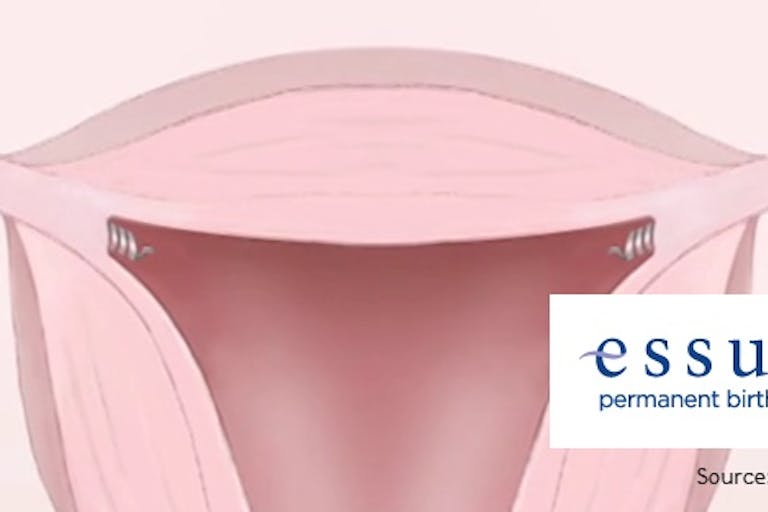
Study: FDA used faulty data when it approved Essure
According to a new study out of Northwestern University, the US Food and Drug Administration has used faulty data in its approval process for a variety of products over the past fifteen years, including the Essure sterilization device.
“Our results reveal significant weaknesses in the preapproval and post approval regulation of high-risk obstetrics and gynecology devices,” the authors said.
As Live Action News has previously covered, objections to Essure on safety grounds have come from both the pro-life and pro-choice camps. The device has been the subject of more than 5,000 complaints to the FDA, which has been accused of downplaying its effects, which include both debilitating harm to women and deaths of babies conceived despite their mothers using the device.
According to LifeSiteNews, the study’s researchers noted:
Of the 18 ob-gyn devices reviewed for the study, six of them, or 33 percent, were not required to go through post-market studies to examine ongoing safety.
Four of the devices, or 22 percent, were approved despite failing to demonstrate effectiveness in clinical trials.
Three devices were ultimately withdrawn from the market after approval. Of those three, two had not been reviewed by the FDA’s obstetrics and gynecology advisory committee’s physician experts, and the one that was reviewed by the committee did not get its recommendation for approval.
The FDA approved Essure in 2002, but according to the researchers it only did so on evidence pertaining to short-term effects and without adequate follow-up once it was on the market.
“There are no explicit requirements about conducting randomized-controlled trials or post-market surveillance for medical devices,” Dr. Steve Xu said, noting that such devices are subject to much looser scrutiny than drugs, with review requirements decided on a case-by-case basis.
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Bridget Sielicki
·
Politics
Cassy Cooke
·
International
Cassy Cooke
·
International
Bridget Sielicki
·
Human Interest
Bridget Sielicki
·
Politics
Nancy Flanders
·
Politics
Calvin Freiburger
·
Guest Column
Calvin Freiburger
·
Abortion Pill Reversal
Calvin Freiburger
·
Guest Column
Calvin Freiburger
·
Abortion Pill Reversal
Calvin Freiburger
·