Missouri, Kansas, and Idaho have filed an amended lawsuit in federal court, claiming their “sovereign” interest in “protecting… citizens” was violated by the Food and Drug Administration’s (FDA) decision to expand access to the abortion pill to mail-order dispensing.
The lawsuit, State of Missouri; State of Kansas; State of Idaho v. FDA, filed on October 14, 2024, in Amarillo, Texas, is “asking US District Judge Matthew Kacsmaryk to rollback efforts the Food and Drug Administration has taken over the past eight years to ease access to the drug, such as allowing it to be dispensed through the mail,” CNN reported.
The Plaintiff States allege that “Women face severe, even life-threatening, harm because the federal government has disregarded their health and safety. These dangerous drugs are now flooding states like Missouri and Idaho and sending women in these States to the emergency room.”
As such, the lawsuit also alleged that “Defendant U.S. Food and Drug Administration (FDA) has the statutory responsibility to protect the health, safety, and welfare of all Americans by putting commonsense safeguards on high-risk drugs[;] the FDA has failed in this responsibility by removing many of the safety standards it once provided to women using abortion drugs.”

Three state Missouri Kansas Idaho v FDA lawsuit on abortion pill
The State of Idaho, along with Missouri AG Andrew Bailey and Kansas AG Kris W. Kobach, brought the lawsuit to “vindicate [their] sovereign, quasi-sovereign, and proprietary interests, including its interests in protecting its citizens,” the court document read.
States request preliminary injunction to reinstate prior safety regulations
In their lawsuit, the Plaintiff States request a “preliminary injunction” from the Court against the FDA, or “a stay of the effective dates” that “reinstates the REMS that were in place before 2016 insofar as they restore the Day 3 and Day 14 follow-up visits, restore the gestational age to 7 weeks from 10 weeks, restore the requirement that prescribers be physicians, and restore the requirement that prescribers must report all serious non-fatal adverse events to the agency; rescinds the 2019 generic approval; and restores the in-person dispensing requirement.”
The three states are asking the court to “issues[] a permanent injunction ordering Defendants to withdraw Defendants’ actions to deregulate these abortion drugs.”
The abortion pill Mifeprex (mifepristone) was approved by the FDA in September 2000 “for medical termination of pregnancy,” the FDA website states. “FDA approved a generic version of Mifeprex, Mifepristone Tablets, 200 mg, in April 2019.”
In 2016, the Obama administration FDA weakened the safety requirements (REMS) for the drug by removing requirements that women take the first drug in front of a clinician in person, at the location of a certified prescriber. It also expanded use of the drug to abort preborn children up to 10 weeks (70 days) of pregnancy from the previous seven weeks, and removed the requirement for the manufacturer (Danco, and now GenBioPro) report the drug’s non-fatal adverse events (complications). Only deaths would be required for reporting.
By December of 2021, the Biden-Harris FDA had further weakened the REMS by eliminating the in-person dispensing requirement and enabling the abortion pill to be permanently shipped by mail. Then in January 2023, the Biden-Harris FDA further gutted the REMS by announcing it would allow retail pharmacies to dispense the drug.
The Plaintiff States claim that the “FDA made [these changes] without any studies that evaluated the impact of removing all of these interrelated safeguards at once.” The suit adds that the undoing of the requirement to report “non-fatal complications” was “unreasonable” because it was “based on past data collected under the originally approved safety standards, not the new deregulated regime.”
The Plaintiffs also noted:
Women face severe bleeding, ruptured ectopic pregnancies, and life threatening infections because the FDA recklessly removed in-person safety standards that it once provided. Women should have the in-person care of a doctor when taking high-risk drugs.
The States of Missouri, Kansas, and Idaho thus challenge the FDA’s actions to remove commonsense safety measures for abortion drugs and ask that the Court hold these actions unlawful, stay their effective date, set them aside, and vacate them.
In rolling back safeguard after safeguard, the FDA has turned a blind eye to the known harms of abortion drugs to the detriment of women and girls.
States seek enforcement of Comstock Law, which prohibits mailing abortion drugs
In their complaint, the Kansas, Missouri, and Idaho are seeking to enforce a law (18 U.S.C. § 1461), known as the Federal Comstock Act, which prohibits the mailing of “any article, instrument, substance, drug, medicine, or thing [that] may, or can, be used or applied for producing abortion[.]”
They claim that “[t]he FDA’s 2021/2023 Removal of the In-Person Dispensing Protection violates the federal laws that expressly prohibit the mailing or delivery by any letter carrier, express company, or other common carrier, or by interactive computer service, of any substance or drug intended for producing abortion” by “impermissibly remov[ing] the in-person dispensing requirement for abortion drugs and, accordingly, authorized the downstream distribution of abortion drugs by mail, express company, other common carriers, and interactive computer service.”
“Because a federal agency cannot permit what federal law expressly prohibits, the FDA lacked legal authority when issuing the 2021/2023 Removal of the In-Person Dispensing Protection,” the lawsuit claimed. That decision “failed to account for or address the federal laws that prohibit the distribution of abortion drugs by postal mail, express company, or common carrier and by interactive computer service,” the lawsuit notes. “FDA permitted and sometimes even encouraged these illegal activities. But a federal agency cannot authorize unlawful actions.”
States argue their standing to bring the lawsuit
Kansas, Missouri, and Idaho are clear that the “FDA’s actions interfere with Plaintiff States’ “sovereign interest in ‘the power to create and enforce a legal code.”
State of Missouri; State of Kansas; State of Idaho v. FDA follows a June 2024 ruling by the United States Supreme Court in a separate case, FDA vs. Alliance for Hippocratic Medicine (AHM). The latter challenged as unsafe the FDA’s expansions of abortion pills in 2016 and 2021 — from in-person visits with a lower gestational limit and more safeguards, to mail-order (and now pharmacy) dispensing. The doctors testified to having treated women with severe complications — “many who presented to the emergency room.”
While Plaintiff doctors in AHM also requested that the Supreme Court reinstate the FDA’s pre-2016 REMS safety requirements for mifepristone, calling the recent expansions of the drug “arbitrary, capricious, an abuse of discretion, and otherwise unlawful,” the Court’s decision in AHM merely ruled that the plaintiff doctors in the AHM lawsuit did not have standing to sue.
Referring to the new lawsuit, CNN wrote that “the states claim they have standing to sue because the FDA’s actions facilitate violations of state abortion laws ‘by enabling an out-of-state abortion drug distribution network.’ The states also claimed the FDA’s moves displaced state laws controlling abortions for girls in foster care.”
The three states alleged in their lawsuit that they have standing to sue “based on (1) federal assertions of authority to regulate matters they believe they control, (2) federal preemption of state law, and (3) federal interference with the enforcement of state law, at least where ‘the state statute at issue regulates behavior or provides for the administration of a state program’ and does not ‘simply purport to immunize state citizens from federal law.’ They also claim to have “suffered injury to their sovereign interests in enacting and enforcing their laws.”
They say the FDA “intentionally facilitated widespread violations by third parties of state abortion laws..[,] unlawfully removed the backstop of federal law and federal law enforcement..[,] purport to preempt state abortion laws, and (4) seek to displace and nullify the States’ state-law parental rights of notice and consent for abortions for teen girls in foster care.”
States argue “abortion drugs are dangerous”
Before Roe v. Wade, so-called self-administered abortions were portrayed by abortion advocates as dangerous. But once Big Abortion began toying with the idea of “no-test” abortion pill protocols and mail-order telabortion schemes (including an over-the-counter abortion pill push), suddenly, self-managed abortion became “safe.”
Or so Big Abortion claims.
The three Plaintiff states disagree, writing in their complaint, “Abortion drugs are dangerous—the FDA’s own label says that an estimated roughly one in 25 women who take abortion drugs will visit the emergency room.”
According to the lawsuit, “[] the FDA has enabled online abortion providers to mail FDA approved abortion drugs to women in states that regulate abortion—dispensing abortion drugs with no doctor care, no exam, and no in-person follow-up care. These dangerous drugs are now flooding states like Missouri and Idaho and sending women in these States to the emergency room.”
Emergency visits as a result of the abortion pill
Live Action News has previously documented that mifepristone’s 2023 label still contains a black box warning for sepsis, bleeding, and other life-threatening risks.
In addition, published percentages for emergency room (ER) visits on the drug’s insert indicate that 2.9 to 4.6 percent of women who take abortion drugs end up in the emergency room, indicating that abortion pill ER visits could be in the tens of thousands every year. In addition, the FDA’s medication guide acknowledges that as many as seven percent (7%) of women will need surgery after taking mifepristone “to stop bleeding” or to complete the abortion.
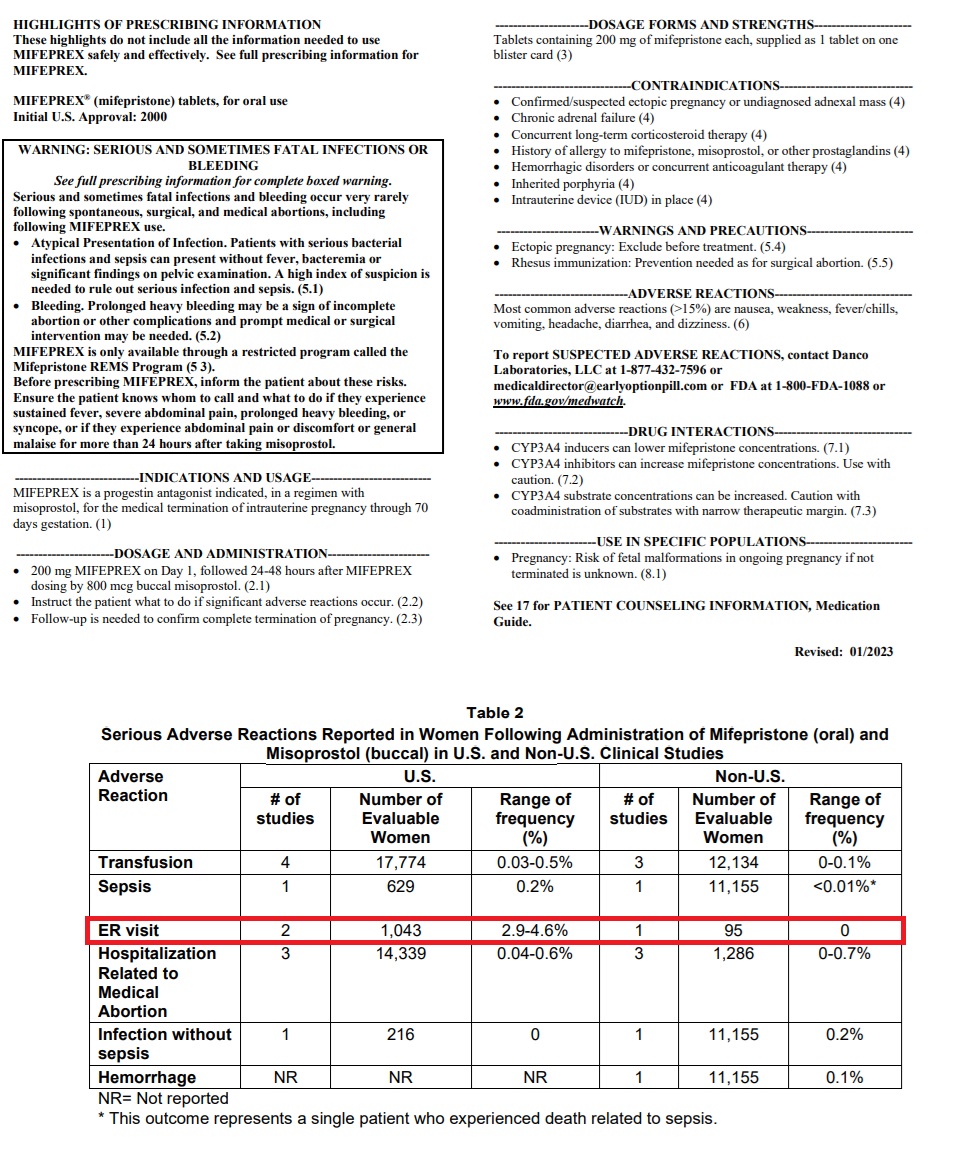
Mifepristone Jan 2023 label shows percentage of women taking abortion pill visit ER
The data also is similar to findings from a previously-documented Gynuity Health Projects (GHP) telabortion study, which found that six percent (6%) of participants (70 out of 1,157) faced complications from the abortion pill, resulting in ER or urgent care visits. Gynuity is a pro-abortion group, conducting clinical trials on the abortion pill, and funded by organizations with deep historic ties to the American eugenics movement.
Abortion pill complications have been offloaded to emergency rooms instead of prescribers
Live Action News has previously documented that the risks of the abortion pill triggered the FDA to place it under a safety system called REMS, where it has remained — even under multiple pro-abortion administrations.
Under the REMS, prescribers of the drug are supposed to have provisions to handle abortion pill failures or complications. Instead of abiding by the regulations, which are policed by those who profit from the drug (Danco and GenBioPro), abortion providers instead simply instruct women to present to the ER.
In other words, they are offloading their abortion pill clients onto already potentially overcrowded emergency rooms.
Live Action News previously documented in detail how Danco and the FDA always understood that emergency rooms would be necessary to treat abortion pill clients, even as the FDA allowed for the expansion of “self-managed” and “mail-order” abortion — something the Plaintiffs alluded to when they wrote: “The FDA has consistently identified emergency medical care—including State emergency medical care—as the backstop for abortion drug complications. Its current label directs women to emergency rooms if one of many adverse complications arise.”
“The FDA has acted unlawfully,” the three states allege. “Now, the State Plaintiffs ask the Court to protect women by holding unlawful, staying the effective date of, setting aside, and vacating the FDA’s actions to eviscerate crucial safeguards for those who undergo this dangerous drug regimen.”
A “no-test protocol” can lead to increased rates of incomplete abortion
“The complications of abortion drugs increase as the baby’s gestational age increases. One study found that, after nine weeks’ gestation, almost four times as many women and girls experience an incomplete abortion, nearly twice as many suffer an infection, and over six times as many women and girls require surgical abortion after consuming the abortion drugs than at before nine weeks gestation,” reads the states’ complaint (emphasis added).
Live Action News has previously documented how the abortion industry used the COVID-19 pandemic to lift the in-person safety regulations on mifepristone, expanding access to it. In fact, well before the pandemic, the abortion industry expanded its abortion pill clinical trials and then rolled out a “no test” abortion pill protocol — eliminating important testing, bloodwork, and ultrasounds, which some medical professionals assert endangers women.
“The risks of harm to women are also exacerbated without follow-up visits, during which a doctor can assess whether a mother is suffering complications from the older gestational age of her baby,” the lawsuit added.
Danco Laboratories, the drug’s manufacturer, claims online that “Mifeprex*(mifepristone) is 93-98% effective for safely ending pregnancy (2-7% of women will need a surgical procedure to end the pregnancy or stop heavy bleeding).”
In addition, Planned Parenthood’s national website is clear that “At 10-11 weeks pregnant” the abortion pill regimen only “works about 87% of the time.”

Danco abortion pill efficacy rates accessed 10182024
Despite this fact, Big Abortion now prescribes the abortion pill past the FDA approved 10-week limit.
In addition, one Planned Parenthood facility admitted online that “After 11 weeks, there’s a bigger chance that the abortion pills won’t work and an increased chance of stronger bleeding or cramps.” Planned Parenthood’s website says little about the effectiveness of the abortion pill at 12 weeks.
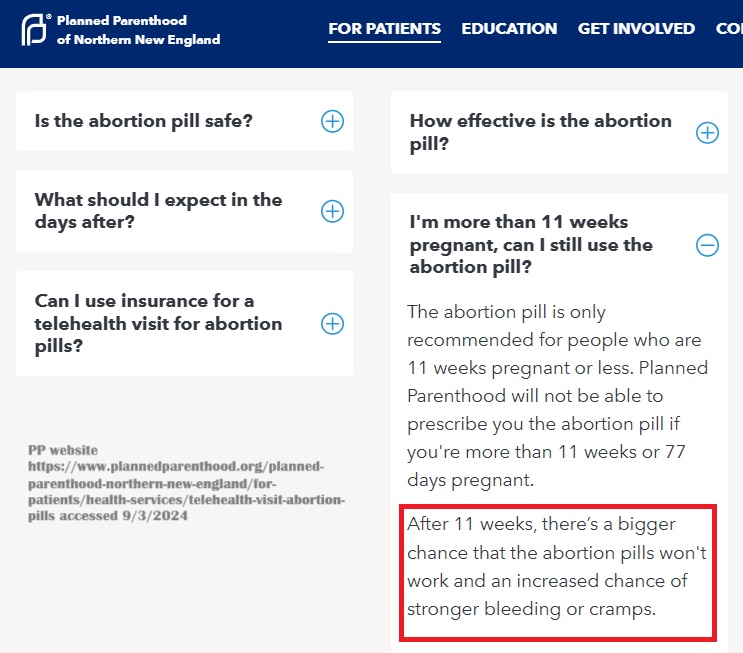
Planned Parenthood New England warns abortion pills less likely to work after 11 weeks
A “no-test” protocol also increases risks for Rh-negative women
“Abortion drugs present heightened risks for women with an Rh-negative blood type. If these women are not administered Rhogam at the time of their chemical abortion, they may experience isoimmunization, which threatens their ability to have future successful pregnancies,” the lawsuit claims.
The American College of Obstetricians and Gynecologists (ACOG) changed its recommendations to coincide exactly with the abortion industry’s attempts to expand access to the abortion pill. Then, just weeks ago, ACOG once again co-signed with Big Abortion against the health of women, regarding when to administer RhoGAM.
OBGYN Dr. Ingrid Skop warned this move is dangerous, explaining to Live Action News (emphasis added), “Evaluation of Rh status and provision of Rhogam, if indicated, has long been the standard of care for early pregnancy loss, including elective induced abortion. This action will prevent a mother from mounting an immune response to her future unborn children. If Rhogam is not given and isoimmunization occurs, 14% of untreated infants will be stillborn and half will suffer neonatal death or brain injury.”
The abortion industry is failing to care for women
The abortion industry has shifted responsibility for abortion pill clients from abortion providers to overcrowded emergency rooms. In addition, bad actors inside the abortion industry continue to flout the FDA’s REMS by ignoring the approved gestational limits or approved protocols for prescribing the abortion pill; leaving women to deal with ectopic pregnancies by not ruling them out prior to prescribing the drug; promoting an unapproved one-drug regimen of misoprostol only; dispensing abortion drugs to women who are not yet pregnant; and encouraging women to present to the emergency room and claim a natural miscarriage when experiencing abortion pill related complications.
This flouting of the FDA REMS safety requirements may have prompted well-known late term abortionist Warren Hern to recently question the care women receive when they are sold abortion pills by asking the obvious: Who is doing “follow-up exam[s]” and “tak[ing] care of… a complication?”
Due to a lack of care and the so-called ‘no-test‘ abortion pill protocol which often fails to utilize ultrasounds, run labs, or rule out potentially dangerous ectopic pregnancies, medical doctors are now alerting ER professionals that they may see undiagnosed ectopic pregnancies after abortion pill use. This is due to a reckless lack of concern abortion providers have in relying on the woman, showing they remain pregnant after taking the deadly drug regimen rather than ruling ectopics out first.
Today, Google Reviews from clients purchasing abortion pills at various facilities suggest that some prescribers of the drug are failing to properly treat women, resulting in them seeking emergency medical assistance ERs.
“The FDA’s actions force States to divert resources to investigate and address the harms that this lawbreaking will inflict on women, children, and the public interest…The FDA’s actions thus ‘intrude on state governmental functions[,]’…and hobble States’ efforts to protect health and safety,” wrote Missouri, Kansas, and Idaho.







