
Ireland lawmakers vote down 'radical' abortion expansion bill
Bridget Sielicki
·
Abortion Pill·By Carole Novielli
FACT CHECK: The Supreme Court never ‘approved’ abortion pill or ruled on ‘access’ to it
UPDATE, 11/21/24: Reuters notes that the Alliance for Hippocratic Medicine and other Plaintiffs have made the decision to drop their lawsuit challenge regarding the abortion pill after the Supreme Court ruled that they did not have standing to sue; three states have picked up the suit — Idaho, Missouri, and Kansas. These states “argu[e] that they were harmed when their state Medicaid programs had to pay to treat women for complications from taking mifepristone, and seeking reimpose the pre-2016 restrictions.”
Reuters wrote:
While the plaintiffs could have tried to revive their case with new evidence and arguments supporting their standing, they would likely have faced an uphill battle after the Supreme Court’s decision. They did not give a reason for dismissing their case, and did so without prejudice meaning they could refile it in the future.
Julie Blake, senior counsel at Alliance Defending Freedom, which represents the plaintiffs, in a statement said that the group “commends the three states that intervened in the case as they continue to seek to hold the FDA accountable.”
7/17/24: Despite recent claims, the United States Supreme Court has neither “approved” the abortion pill, nor ruled on whether the “American people should have access” to the abortion pill regimen.
SCOTUS Blog, in the title of its article about the ruling, claimed that the Court “preserved access” to the abortion pill. Healthline, in an allegedly “fact checked” and verified article, claimed the “Supreme Court Rule[d] to Preserve Access” to the abortion pill, and also stated, “On June 13, the U.S. Supreme Court ruled in favor of the abortion pill mifepristone after weighing whether to restrict access,” and “The U.S. Supreme Court voted unanimously on June 13 that the commonly prescribed abortion medication mifepristone will remain available” (emphases added), before adding what actually happened: “The FDA vs. Alliance for Hippocratic Medicine ruling found that the plaintiffs lacked legal standing to challenge how the FDA regulates mifepristone.”
As Live Action News will document below, the Supreme Court merely made the “decision” that the plaintiff doctors who brought the lawsuit did not have standing to sue. The Supreme Court did not “rule” anything about “access” to the abortion pill.
Trump and Vance Questioned by Media about Abortion Pill
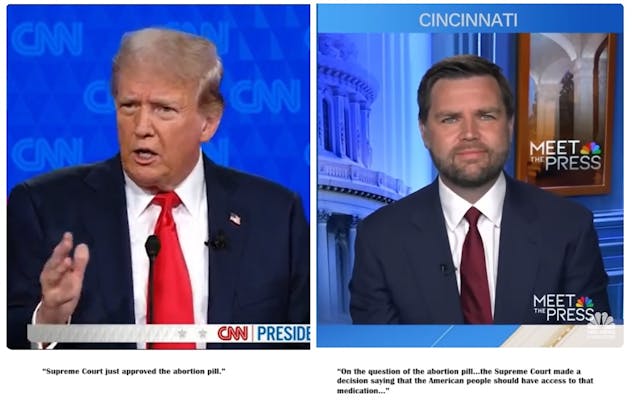
In the first presidential debate of 2024 between president Joe Biden and former president Donald Trump, debate moderator Dana Bash asked Trump whether or not he would ban access to the abortion pill.
“The Federal Government still plays a role in whether or not women have access to abortion pills… As President, would you block abortion medication?” Bash asked Trump.
“First of all, the Supreme Court just approved the abortion pill and I agree with their decision to have done that, and I will not block it,” Trump responded.

Then, in a July 7 interview with Senator J.D. Vance on NBC News’ Meet the Press, host Kristen Welker told Vance that “Donald Trump says he supports access to that pill, actually.”
She then asked the Senator, “Do you support access to abortion medication as Donald Trump does?”
In response, Senator Vance incorrectly summarized the Supreme Court decision in the Alliance for Hippocratic Medicine (AHM) versus Food and Drug Administration (FDA) lawsuit, claiming the high court granted Americans “access” to the abortion pills.
“On the question of the abortion pill what so many of us have said is look, the Supreme Court made a decision saying that the American people should have access to that medication. Donald Trump has supported that opinion, I support that opinion,” Senator Vance stated. “I think it’s important to say we actually have to have the important conversation in this country about what our abortion policy should be. Donald Trump is the pragmatic leader here. He’s saying most abortion policy is going to be decided by the states….”
What the Supreme Court DIDN’T Do
These analyses of the Supreme Court abortion pill decision were incorrect.
President Donald Trump: “the Supreme Court just approved the abortion pill.”
Senator J.D. Vance: “On the question of the abortion pill…the Supreme Court made a decision saying that the American people should have access to that medication…”
The case Senator Vance and President Trump were referring to was initiated in November 2022 by the Alliance for Hippocratic Medicine (AHM), a group of medical doctors and organizations who challenged as unsafe the FDA’s expansions of abortion pills in 2016 and 2021. The doctors testified to treating women with severe complications — “many who presented to the emergency room.”
AHM’s lawsuit requested that the Supreme Court reinstate the FDA’s pre-2016 REMS safety requirements for mifepristone, calling the recent expansions of the drug “arbitrary, capricious, an abuse of discretion, and otherwise unlawful.”
However, the Court never ruled on the merits of the AHM lawsuit against the FDA. The Supreme Court’s unanimous decision in the case centered solely on whether the Plaintiff doctors who filed the lawsuit had legal standing to sue.
What the Supreme Court actually did
Through a series of legal hearings, a 62-page decision issued by the appeals court in 2023 ruled to allow mifepristone (the abortion pill) to remain “available to the public under the conditions for use that existed” prior to 2016. Shortly thereafter, in December of 2023, the Supreme Court announced that it would hear the case. In June of this year, the Supreme Court issued its ruling.
Article continues below
Dear Reader,
In 2026, Live Action is heading straight where the battle is fiercest: college campuses.
We have a bold initiative to establish 100 Live Action campus chapters within the next year, and your partnership will make it a success!
Your support today will help train and equip young leaders, bring Live Action’s educational content into academic environments, host on-campus events and debates, and empower students to challenge the pro-abortion status quo with truth and compassion.
Invest in pro-life grassroots outreach and cultural formation with your DOUBLED year-end gift!
“The [District] court first held that the plaintiffs possessed Article III standing. It then determined that the plaintiffs were likely to succeed on the merits of each of their claims. Finally, the court concluded that the plaintiffs would suffer irreparable harm from FDA’s continued approval of mifepristone and that an injunction would serve the public interest,” Justice Brett Kavanaugh wrote in the June 2024 Supreme Court decision regarding access to abortion pills. He then went onto address the issue of standing.
“The fundamentals of standing are well-known and firmly rooted in American constitutional law. To establish standing, as this Court has often stated, a plaintiff must demonstrate (i) that she has suffered or likely will suffer an injury in fact, (ii) that the injury likely was caused or will be caused by the defendant, and (iii) that the injury likely would be redressed by the requested judicial relief,” the Supreme Court opinion stated.
“Because the plaintiffs do not prescribe, manufacture, sell, or advertise mifepristone or sponsor a competing drug, the plaintiffs suffer no direct monetary injuries from FDA’s actions relaxing regulation of mifepristone. Nor do they suffer injuries to their property, or to the value of their property, from FDA’s actions. Because the plaintiffs do not use mifepristone, they obviously can suffer no physical injuries from FDA’s actions relaxing regulation of mifepristone,” the opinion added, also noting that “The plaintiffs have not identified any instances where a doctor was required, notwithstanding conscience objections, to perform an abortion or to provide other abortion-related treatment that violated the doctor’s conscience since mifepristone’s 2000 approval.”
The opinion added that “…the causal link between FDA’s regulatory actions in 2016 and 2021 and those alleged injuries is too speculative, lacks support in the record, and is otherwise too attenuated to establish standing.”
In other words, the Supreme Court did not even rule on the question of whether the FDA acted lawfully when the government agency expanded access to the drug to eliminate the in-person requirement and enable mail order and pharmacy dispensing, among other measures.
Instead, the Court merely ruled that the AHM doctors did not have standing to bring the lawsuit in the first place.
63% of Abortions Now Done by Abortion Pill
The abortion pill was approved in 2000 as mifepristone (Mifeprex) and is administered in a 200-milligram dosage. It is manufactured under the direction of Danco Laboratories, and the generic version under GenBioPro.
In 2016, the Food and Drug Administration (FDA) weakened the FDA’s safety regulations (REMS), removing in-person requirements for prescribing the drug, extending use of the drug on preborn children up to 10 weeks (70 days) of pregnancy, and removing the requirement that the manufacturer report the drug’s non-fatal adverse events (complications). In more recent years, the Biden FDA has expanded access to the abortion pill to include mail-order and pharmacy dispensing.
In March of 2024, the Guttmacher Institute estimated that the abortion pill accounted for 63% of abortions in 2023 — a 45% increase from 2017, as previously reported. That data equated to 642,700 chemical (abortion pill) abortions in 2023. This translates to an abortion pill count of 53,558 monthly, 1,761 daily, 73 hourly, and one abortion by pill every 49 seconds.
The latest published data by the FDA revealed that from 2000 through the end of December 2022, “approximately 5.9 million women” used the abortion pill mifepristone in the U.S. for “medical termination of pregnancy.”
In addition to these chemical abortion deaths, the FDA reports 32 deaths* “associated with mifepristone” since its approval in 2000.
Case Challenging Abortion Pill Far From Over
Following the Supreme Court’s June 2024 ruling, the Alliance Defending Freedom (ADF), which represented the AHM plaintiffs, suggested in a press conference that the legal case could potentially continue at the lower court level, as three other states (Idaho, Kansas, and Missouri) have initiated similar lawsuits.
This point was also made by SCOTUS Blog, which wrote that the Supreme Court’s “ruling means that mifepristone will continue to remain widely available in the United States, where it is used in over 60% of abortions by health care providers. The decision, however, does not necessarily foreclose another challenge to the FDA’s actions. Three states with Republican attorneys general – Idaho, Missouri, and Kansas – joined the dispute in the lower court earlier this year.”
“The case now returns to the lower courts, and the dispute over access to the drug likely is not over,” SCOTUS Blog stated.
Nancy Northrup, Center for Reproductive Rights president and CEO, was quoted as warning that access to mifepristone “is still at risk nationwide.”
Despite the incorrect statements, Live Action News has yet to locate any corrections published regarding the Supreme Court AHM case, nor have any mainstream media outlets fact-checked these erroneous claims.
Editor’s Note, 7/31/24: Additional links have been added showing the media’s incorrect reporting on the SCOTUS ruling.
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Bridget Sielicki
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Bridget Sielicki
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Bridget Sielicki
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Human Rights
Carole Novielli
·