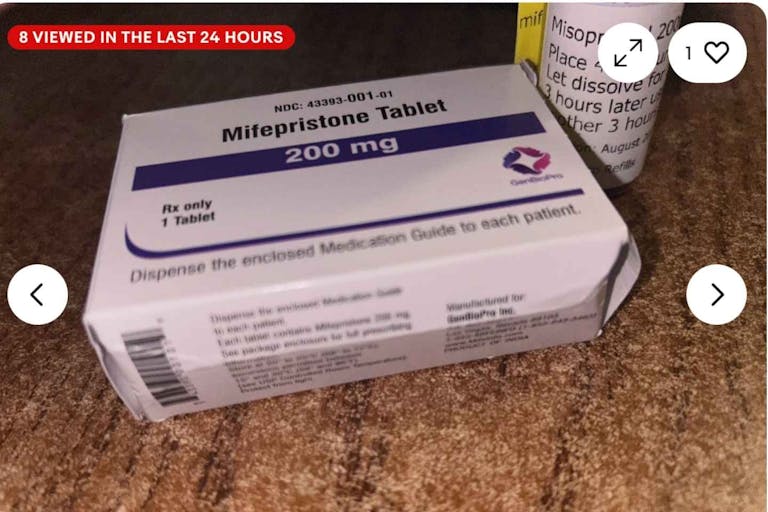
This eBay store is selling the abortion pill, putting women in danger
Carole Novielli
·
Pro-life leaders urge FDA to crack down on illegal sale of abortion pills, keep safeguards in place
Today, 56 pro-life leaders sent a letter to the Food and Drug Administration, asking the agency to crack down on websites illegally selling the abortion pill.
Multiple sites such as AidAccess continue to operate despite the FDA’s Risk Evaluation and Mitigation Strategy, known as REMS, which requires certain safeguards surrounding the distribution of the abortion pill in the United States. As stated in a press release from the Susan B. Anthony List, a pro-life public policy organization, “Under the FDA’s Risk Evaluation and Mitigation Strategy (REMS), the abortion drug Mifeprex or generic mifepristone cannot legally be sold in pharmacies or online due to the risk of serious complications – but website domains such as AidAccess and Rablon that sell and distribute abortion-inducing drugs continually fail to comply with REMS requirements.”

While the FDA sent warning letters to such companies in 2019, AidAccess openly announced its intention to defy the FDA. A Live Action News article noted:
In May 2019, the FDA told Live Action News, “We cannot comment on a potential future action at this time, but we remain very concerned about the sale of unapproved mifepristone for medical termination of early pregnancy on the Internet, because this bypasses important safeguards designed to protect women’s health. Unapproved drugs purchased from foreign internet sources are not the FDA-approved versions of the drugs, and therefore, they are not subject to FDA-regulated manufacturing controls or FDA inspection of manufacturing facilities. Drugs that have circumvented regulatory safeguards may be contaminated, counterfeit, contain varying amounts of active ingredients, or contain different ingredients altogether.”
Since that time, while the FDA has been cracking down on the illegal distribution of other substances, no action has been taken against illicit abortion pill vendors.
SBA List president Marjorie Dannenfelser noted in SBA List’s press release, “Chemical abortion drugs pose a serious risk of potentially life-threatening complications. Research shows as many as five to seven percent of women who undergo chemical abortions will require follow-up surgery. While the abortion industry and its allies promote these drugs as the safe, easy, near painless way to have an abortion, the truth is that women have experienced intense pain, severe and heavy bleeding, and even death as a result of chemically-induced abortions.”

Article continues below
Dear Reader,
In 2026, Live Action is heading straight where the battle is fiercest: college campuses.
We have a bold initiative to establish 100 Live Action campus chapters within the next year, and your partnership will make it a success!
Your support today will help train and equip young leaders, bring Live Action’s educational content into academic environments, host on-campus events and debates, and empower students to challenge the pro-abortion status quo with truth and compassion.
Invest in pro-life grassroots outreach and cultural formation with your DOUBLED year-end gift!
During COVID-19, the abortion industry has ramped up its push for “self-managed” abortion, urging the FDA to lift REMS so that women won’t have to visit an authorized provider to receive abortion pills. But the FDA recently told VICE in response to the calls to lift REMS, “Certain restrictions, known as a risk evaluation and mitigation strategy (REMS), are necessary for mifepristone when used for medical termination of early pregnancy in order to ensure that the benefits of the drug outweigh its risks.”
READ: Group vows to defy FDA, continues dispensing abortion pills illegally
The industry, however, has been finding ways to skirt these safety protocols, specifically through a rapidly expanding clinical trial known as TelAbortion. Participants in the trial may be as young as 10, and will have abortion pills sent to them through the mail. The abortion pill is currently distributed in a few different ways — through telemedicine (in which the client must visit a facility where she speaks with a remote provider and then receives the abortion pills), through the TelAbortion trial already described, and through something known as “no-test” or “no contact” abortion, in which no testing of any kind (ultrasound, blood test, etc.) is required for a client to receive the abortion pill. Not even an accurate gestational age will be assessed by the provider under the “no-test” protocol. The dangers to women are grave. See the graphic below for a short summary:

In their letter to the FDA, pro-life leaders note, “We appreciate that the FDA is seriously defending the necessary safeguards contained in the Mifeprex REMS litigation. These safeguards are meaningless, however, if opportunistic entities can sell abortion-inducing drugs over the internet with impunity. We urge the FDA to act now to stop this predatory and dangerous practice.”
The pro-life leaders and organizations signing on to the letter include:
Marjorie Dannenfelser, President of SBA List; Dr. Donna J. Harrison M.D., Executive Director of the American Association of Pro-life Obstetricians and Gynecologists; Bradley Mattes, President of Life Issues Institute; Kristan Hawkins, President of Students for Life of America and Students for Life Action; Catherine Glenn Foster, President and CEO of Americans United for Life; Jeanne Mancini, President of March for Life; Lila Rose, President and Founder of Live Action; Penny Young Nance, President and CEO of Concerned Women for America; Carol Tobias, President of National Right to Life; Dr. Jeffery Barrows D.O. M.A., Senior Vice President of Bioethics and Public Policy at the Christian Medical and Dental Associations; Dr. Kris Held M.D., President of the Association of American Physicians and Surgeons; Fr. Frank Pavone, National Director of Priests for Life; Eunie Smith, President of the Eagle Forum; Thomas A. Glessner, President of the National Institute of Family and Life Advocates (NIFLA); Greg Schleppenbach, Associate Director of the USCCB Secretariat of Pro-life Activities; Jessica Anderson, Executive Director of Heritage Action; Jordan Sekulow, Executive Director of the American Center for Law and Justice; Travis S. Weber, Vice President for Policy and Government Affairs at Family Research Council; Jor-El Godsey, President of Heartbeat International; Russell Moore, President of the Southern Baptist Ethics and Religious Liberty Commission; Dorinda Bordlee, Chief Counsel at the Bioethics Defense Fund; Dr. Ellen Gianoli B.S.N. M.A. R.N., President of the National Association of Catholic Nurses, among others.
“Like” Live Action News on Facebook for more pro-life news and commentary!
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Bridget Sielicki
·
Abortion Pill
Carole Novielli
·
Human Interest
Kelli Keane
·
Activism
Kelli Keane
·
Politics
Kelli Keane
·
Human Interest
Kelli Keane
·
Abortion Pill
Kelli Keane
·