
FACT CHECK: Are pro-life laws to blame for arrest of SC woman who delivered baby in a toilet?
Nancy Flanders
·
Abortion Pill·By Carole Novielli
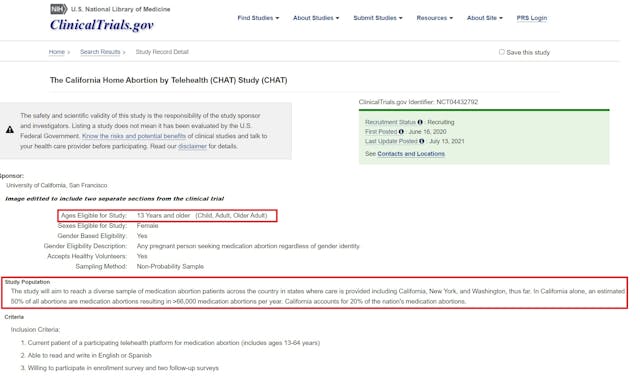
Study: California commits 20% of nation’s total chemical abortions
Nearly 70,000 chemical abortions are committed annually in California (50% of all abortions committed in the state), according to a study sponsored by the University of California San Francisco (UCSF), which trains abortion providers through its Bixby Center for Global Reproductive Health. The information comes from a clinical trial sponsored by UCSF called the CHAT Study, which stands for California Home Abortion by Telehealth.
“CHAT is the first study of its kind in the U.S. that will evaluate abortion medication by mail without the need for a doctor’s visit or ultrasound,” a fundraising page for the study states.
UCSF wrote about the trial, “The study will aim to reach a diverse sample of medication abortion patients across the country in states where care is provided including California, New York, and Washington, thus far. In California alone, an estimated 50% of all abortions are medication abortions resulting in >66,000 medication abortions per year. California accounts for 20% of the nation’s medication abortions.” (emphasis added)
California is not required to report abortions in the state and these numbers are therefore not factored into data published by the Centers for Disease Control and Prevention (CDC), which recently noted that chemical abortions comprised nearly 44% (43.7%, to be exact) of abortions reported to the CDC in 2019.
California has vowed to be a sanctuary for abortion and recently formed the California Future of Abortion Council initiative. In 2019, Live Action News reviewed reports published by Medi-Cal, a California state taxpayer-funded program for low-income children and adults, and found that in just the state’s Fee for Service (FFS) Program, taxpayers paid nearly $700 million for abortions over 25 years, from 1989 to 2014.
California Home Abortion by Telehealth (CHAT) Study
The CHAT study, which it is expected to be completed in October of this year, aims to “assess efficacy and safety outcomes of a telehealth model of abortion care,” and “will compare efficacy of this model to usual in-clinic care based on published rates. It will also investigate participant acceptability and feasibility of this model of care.”
The CHAT study is sponsored by UCSF and is being overseen by Ushma Upadhyay and Jennifer Ko. It is recruiting “females ages 13 years and up.” Upadhyay is an Associate Professor at UCSF’s Advancing New Standards in Reproductive Health (ANSIRH). ANSIRH is part of UCSF’s Bixby Center for Global Reproductive Health. In 2019, well before the COVID-19 pandemic, Upadhyay advocated a “no test/no touch” approach where she and her colleagues suggested that abortion providers should simply “believe the woman” about the exact dating of her pregnancy.
“We will examine medical chart data on all patients from partnered telehealth providers to analyze efficacy and safety outcomes for medication abortion. These medical chart data will include medical/pregnancy history and abortion outcome and de-identified apart from date of birth, zip code, and dates of service,” the sponsors wrote on the trial website.
Then, each telabortion client will receive an optional survey “following completion of the telehealth provider’s standard medical screening.”
READ: California to offer incentives for medical students to become abortionists
The study sponsor claims, “Among those who complete the study surveys, we will investigate the feasibility, time to abortion, efficacy, safety, and acceptability of telehealth provision of mifepristone, measured using a 4-week follow-up though open-ended and closed-ended survey questions. Individuals who opted not to take the medications will be asked a separate set of follow-up questions to collect data related to diversion, to better understand potential risks.”
“With the recent announcement that the FDA is planning to review the REMS requirement, we are at a pivotal moment for the future of abortion care and reproductive autonomy…” Upadhyay previously wrote. “On May 1st 2021, clinical data collection to assess the safety and efficacy of telehealth abortion care commenced. This is the data that the FDA is interested in. We’ll be able to get it in front of them.”
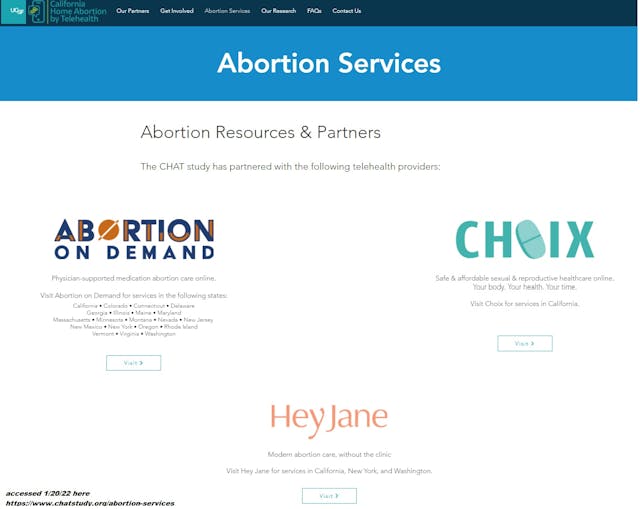
UCSF partners with virtual chemical abortion pill Choix
UCSF is partnering with multiple virtual abortion pill dispensaries to recruit teens and women for their CHAT study, including Choix Inc., HeyJane, and virtual abortion pill website Abortion on Demand (AOD).
In January of 2022, Choix Inc., co-founder Cindy Adam told Ms. Magazine, “There’s also an option to receive a $50 electronic gift card for folks who are interested in participating in a University of California, San Francisco research study.”
“Our chief medical officer is Dr. Aisha Wagner. She provides consultation services for the nurse practitioners as well as clinical protocol development and review. She provides in-person care as a family practice physician and also works for Planned Parenthood doing aspiration abortions,” Adam also said.
Wagner also previously worked for at UCSF’s Women’s Option Center, an abortion facility at the University of California San Francisco.
While Choix’s founders did not elaborate on the UCSF partnership in their Ms. Magazine interview, online their website references the Chat Study, stating, “Researchers at Advancing New Standards in Reproductive Health (ANSIRH), a research group at the University of California, San Francisco, (UCSF), are conducting a study to better understand the experiences of people who use an online service to get an abortion with pills.”
“There are 3 short online surveys that you will take after your regularly scheduled follow ups with a Choix medical provider… After you complete the final survey you will receive a $50 cash card via email within two weeks,” the website also states.
In December of 2021, the Food and Drug Administration (FDA) weakened important safety requirements called REMS on the abortion pill by removing the in-person requirement and enabling the drug regimen to be permanently shipped by mail. The FDA then updated its “Post-Marketing Adverse Events Summary” to show that as of June 30, 2021, the abortion pill has ended the lives of nearly five million babies in the U.S. since its approval in 2000.
“Like” Live Action News on Facebook for more pro-life news and commentary!
Live Action News is pro-life news and commentary from a pro-life perspective.
Contact editor@liveaction.org for questions, corrections, or if you are seeking permission to reprint any Live Action News content.
Guest Articles: To submit a guest article to Live Action News, email editor@liveaction.org with an attached Word document of 800-1000 words. Please also attach any photos relevant to your submission if applicable. If your submission is accepted for publication, you will be notified within three weeks. Guest articles are not compensated (see our Open License Agreement). Thank you for your interest in Live Action News!

Nancy Flanders
·
Abortion Pill
Angeline Tan
·
Abortion Pill
Nancy Flanders
·
Analysis
Cassy Cooke
·
Politics
Bridget Sielicki
·
Human Interest
Andrea Trudden
·
Investigative
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Abortion Pill
Carole Novielli
·
Investigative
Carole Novielli
·
Abortion Pill
Carole Novielli
·